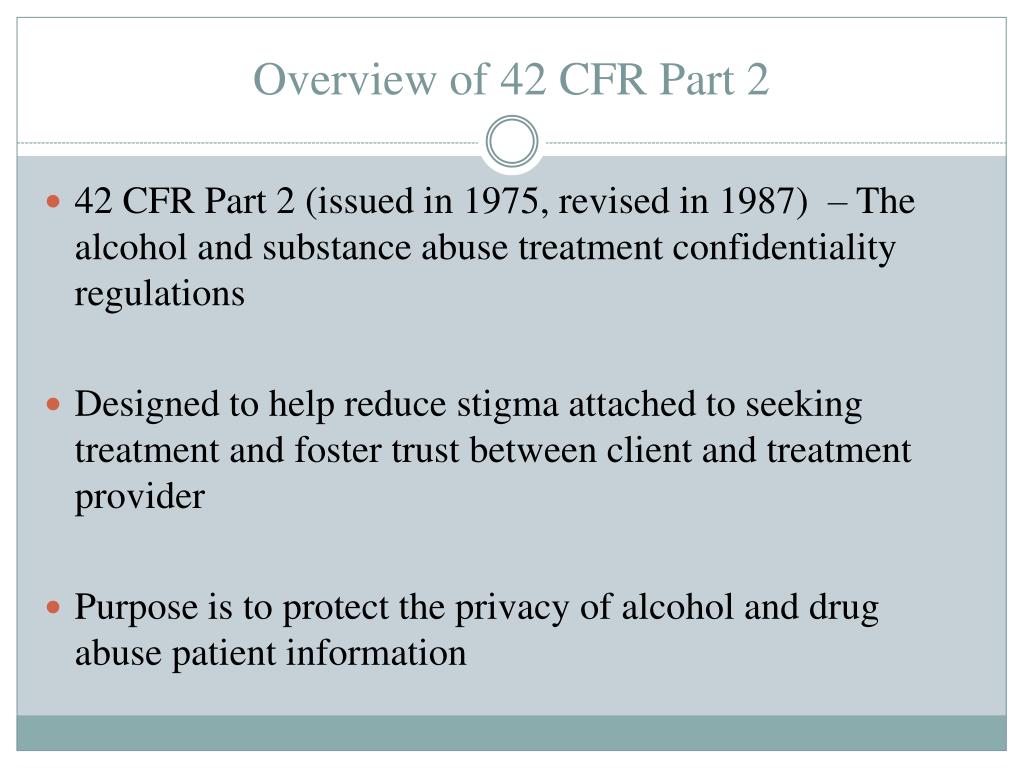
42 Cfr Part 2 Consent Form Sample
42 Cfr Part 2 Consent Form Sample - Web sample consent forms #1 and #2 can be utilized as a guide for grantee programs to either request program participant confidential information from other. (1) the name of the patient. Title 42 was last amended 7/03/2023. Save or instantly send your ready documents. Web confidential substance use disorder information pursuant to 42 cfr part 2, at the offices of coastal carolina neuropsychiatric center, p.a. Please review this form carefully. Easily fill out pdf blank, edit, and sign them. ( 1) the name of the patient. Web title 42 this content is from the ecfr and is authoritative but unofficial. Web complete 42 cfr part 2 consent form sample online with us legal forms.
Web complete 42 cfr part 2 consent form sample online with us legal forms. Web title 42 this content is from the ecfr and is authoritative but unofficial. Web a written consent to a disclosure under the regulations in this part may be paper or electronic and must include: ( 2) the specific name (s) or. ( 2) the specific name (s) or. Web understand that my substance use disorder records are protected under federal law, including the federal regulations governing the confidentiality of substance use disorder. Web a written consent to a disclosure under the regulations in this part may be paper or electronic and must include: Please review this form carefully. Web 4 a full description of the requirements of a part 2 consent form is available at: Web lac uses legal & policy strategies to fight discrimination, build health equity & restore opportunity for people with criminal records, addiction, or hiv.
Web understand that my substance use disorder records are protected under federal law, including the federal regulations governing the confidentiality of substance use disorder. Web sample consent forms #1 and #2 can be utilized as a guide for grantee programs to either request program participant confidential information from other. Web with limited exceptions, 42 cfr part 2 requires patient consent for disclosures of protected health information even for the purposes of treatment, payment, or health care operations. (1) the name of the patient. Developed in compliance with samhsa july 2020 final rule amending 42 cfr part 2. Web 4 a full description of the requirements of a part 2 consent form is available at: Easily fill out pdf blank, edit, and sign them. Authorizing disclosure of confidential sud patient records. Save or instantly send your ready documents. ( 1) the name of the patient.
42 CFR Part 2 Elements of a Valid Consent (5) (1).docx Google Drive
( 2) the specific name (s) or. Web understand that my substance use disorder records are protected under federal law, including the federal regulations governing the confidentiality of substance use disorder. This regulation requires that physicians providing opioid addiction treatment obtain signed patient consent before disclosing individually. ( 1) the name of the patient. Please review this form carefully.
Form C42 Written Authorization printable pdf download
Web 4 a full description of the requirements of a part 2 consent form is available at: (2) the specific name(s) or. Web complete 42 cfr part 2 consent form sample online with us legal forms. Displaying title 42, up to date as of 7/14/2023. Web this consent form allows you to authorize the disclosure of your substance use disorder.
Consent Letter Writing Guide, Types, [+12 Consent Samples]
Please review this form carefully. Displaying title 42, up to date as of 7/14/2023. Authorizing disclosure of confidential sud patient records. Save or instantly send your ready documents. Web lac uses legal & policy strategies to fight discrimination, build health equity & restore opportunity for people with criminal records, addiction, or hiv.
PPT The Interaction Between 42 CFR Part 2 and HIPAA Privacy
Displaying title 42, up to date as of 7/14/2023. Web understand that my substance use disorder records are protected under federal law, including the federal regulations governing the confidentiality of substance use disorder. Web 4 a full description of the requirements of a part 2 consent form is available at: Web complete 42 cfr part 2 consent form sample online.
PPT 42 CFR Part 2 (Substance Abuse Treatment) PowerPoint Presentation
( 2) the specific name (s) or. Web a written consent to a disclosure under the regulations in this part may be paper or electronic and must include: Web lac uses legal & policy strategies to fight discrimination, build health equity & restore opportunity for people with criminal records, addiction, or hiv. This regulation requires that physicians providing opioid addiction.
PPT Ethics and Confidentiality PowerPoint Presentation ID220166
This regulation requires that physicians providing opioid addiction treatment obtain signed patient consent before disclosing individually. Web 10 rows an sud patient may consent to disclosure of the patient’s part 2 treatment records to an entity (e.g., the social security administration), without naming a. ( 2) the specific name (s) or. Displaying title 42, up to date as of 7/14/2023..
Easy Examples of a Therapy Confidentiality Agreement Doolittle Faceing
Web a written consent to a disclosure under the regulations in this part may be paper or electronic and must include: Web confidential substance use disorder information pursuant to 42 cfr part 2, at the offices of coastal carolina neuropsychiatric center, p.a. Web 4 a full description of the requirements of a part 2 consent form is available at: Web.
42 CFR Part 2 The Consent Process
( 2) the specific name (s) or. ( 1) the name of the patient. Web a written consent to a disclosure under the regulations in this part may be paper or electronic and must include: Web title 42 this content is from the ecfr and is authoritative but unofficial. Web a written consent to a disclosure under the regulations in.
PPT The Interaction Between 42 CFR Part 2 and HIPAA Privacy
( 1) the name of the patient. Please review this form carefully. Title 42 was last amended 7/03/2023. Displaying title 42, up to date as of 7/14/2023. Authorizing disclosure of confidential sud patient records.
PPT The Interaction Between 42 CFR Part 2 and HIPAA Privacy
Developed in compliance with samhsa july 2020 final rule amending 42 cfr part 2. ( 1) the name of the patient. Web the federal rules restrict any use of the information to investigate or prosecute with regard to a crime any patient with a substance use disorder, except as provided at §§ 2.12 (c) (5). This regulation requires that physicians.
Easily Fill Out Pdf Blank, Edit, And Sign Them.
🅠 where can i find sample consent forms and redisclosure notices? Web a written consent to a disclosure under the regulations in this part may be paper or electronic and must include: Save or instantly send your ready documents. Web 10 rows an sud patient may consent to disclosure of the patient’s part 2 treatment records to an entity (e.g., the social security administration), without naming a.
Web Confidential Substance Use Disorder Information Pursuant To 42 Cfr Part 2, At The Offices Of Coastal Carolina Neuropsychiatric Center, P.a.
Web lac uses legal & policy strategies to fight discrimination, build health equity & restore opportunity for people with criminal records, addiction, or hiv. ( 2) the specific name (s) or. Web a written consent to a disclosure under the regulations in this part may be paper or electronic and must include: Web sample consent forms #1 and #2 can be utilized as a guide for grantee programs to either request program participant confidential information from other.
(2) The Specific Name(S) Or.
Web a written consent to a disclosure under the regulations in this part may be paper or electronic and must include: ( 1) the name of the patient. Title 42 was last amended 7/03/2023. ( 1) the name of the patient.
Web Title 42 This Content Is From The Ecfr And Is Authoritative But Unofficial.
Web the federal rules restrict any use of the information to investigate or prosecute with regard to a crime any patient with a substance use disorder, except as provided at §§ 2.12 (c) (5). Web this consent form allows you to authorize the disclosure of your substance use disorder records protected by part 2. Displaying title 42, up to date as of 7/14/2023. (1) the name of the patient.

![Consent Letter Writing Guide, Types, [+12 Consent Samples]](https://storage.googleapis.com/fplsblog/consent-form-1/online-consent-form.jpg)