Atoms That Are The Same Form A
Atoms That Are The Same Form A - Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: Web as atoms come together to form molecules, chemical bonds bind them together. An atom is the smallest unit of matter that retains all of the chemical properties of an element. Fundamental properties of atoms including atomic number and atomic mass. Dalton based his theory on the law of conservation of mass and the law of constant composition. So nitrogen has 7 protons and 7 electrons, calcium has 20 protons and 20 electrons, and tin has 50 protons and 50 electrons. The first part of his theory states that all matter is made of atoms, which are indivisible. Web it naturally attracts other atoms with unpaired electrons, such as hydrogen, which has only one electron. Within a single element, the number of neutrons may vary, determining the isotope of that element.
Dalton based his theory on the law of conservation of mass and the law of constant composition. Instead, they’re usually interacting with other atoms (or groups of atoms). The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in. Web elements are made up of particles called atoms. Web the atoms in one element are all the same as each other, but they are different from the atoms of any other elements. In their most common form, many elements also contain the same number of neutrons as protons. The total number of protons and neutrons determine the nuclide. Employing standard techniques, the researchers used laser light to increase and. March 2, 2021 at 6:25 pm. Web home and his team used the shared vibrational motion of two ionized atoms—one calcium and one beryllium—to create their phonon laser.
Atoms can lose or gain electrons. In a simplified model of a water molecule, two atoms of hydrogen share their valence electrons with an atom of oxygen. Web both forms represent the same periodic table. Each atom of hydrogen has two electrons and the. So nitrogen has 7 protons and 7 electrons, calcium has 20 protons and 20 electrons, and tin has 50 protons and 50 electrons. The total number of protons and neutrons determine the nuclide. Some matter is either smaller or larger than an atom.examples of chemical species that are not typically considered atoms includes particles that are components of atoms: Web introduction living things are made up of atoms, but in most cases, those atoms aren’t just floating around individually. The first part of his theory states that all matter is made of atoms, which are indivisible. March 2, 2021 at 6:25 pm.
Are all atoms the same? AMAZING 8TH GRADE SCIENTISTS
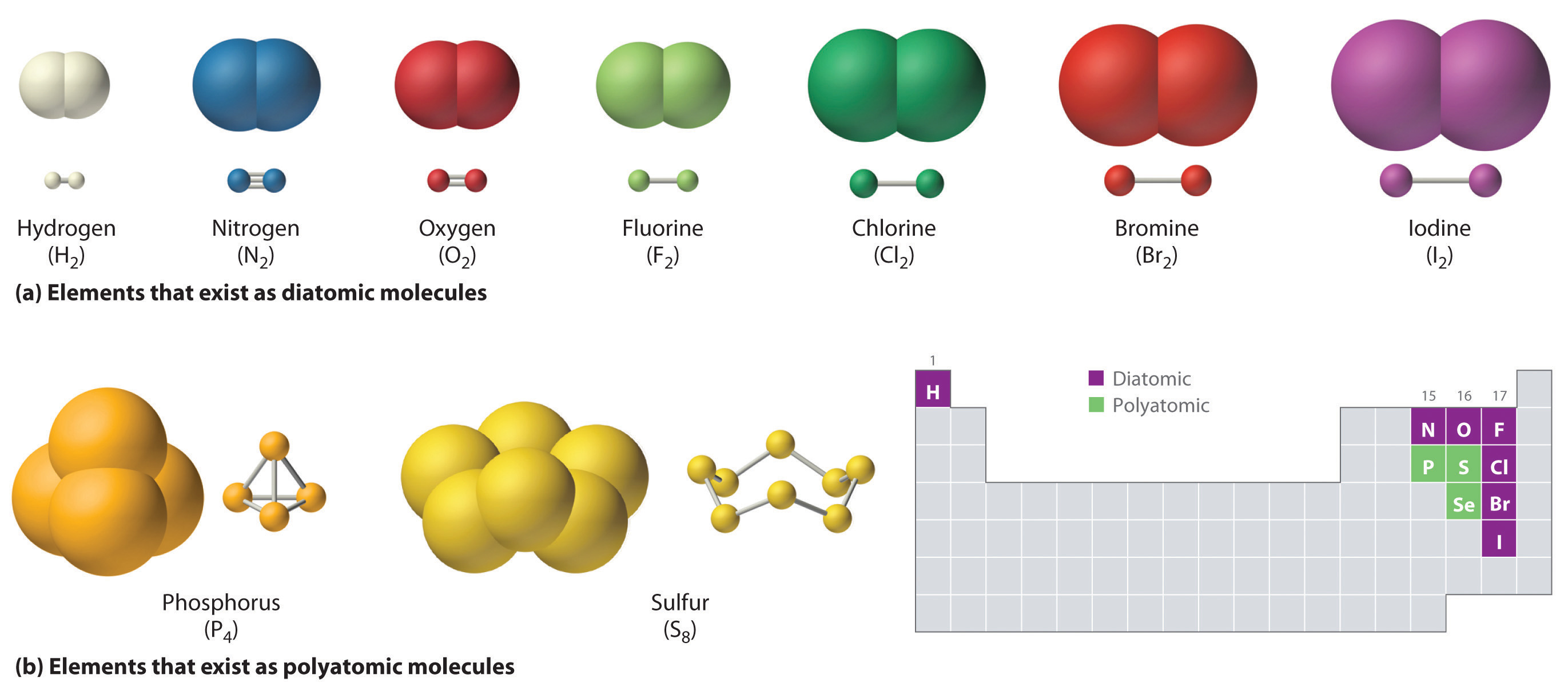
Atoms can lose or gain electrons. If a diatomic molecule consists of two atoms of the same element, such as hydrogen (h 2) or oxygen (o 2), then it is said to be homonuclear. The difference between atom and element. The number of neutrons is very roughly the same as the number of protons, but sometimes it's rather more. Web.
What Is A Half Life? How Elements With Short Half Lives Even Exist?
Web it naturally attracts other atoms with unpaired electrons, such as hydrogen, which has only one electron. When atoms of different elements share electrons through covalent bonding, the electron will be drawn more toward the atom with the higher e lectronegativity resulting in a polar covalent bond. Web atoms with the same number of protons belong to the same chemical.
Molecules and Compounds Definition, Differenences [in Table Form]
The difference between atom and element. For instance, atoms might be connected by strong bonds and organized into molecules or crystals. Web it naturally attracts other atoms with unpaired electrons, such as hydrogen, which has only one electron. The first part of his theory states that all matter is made of atoms, which are indivisible. Atoms can lose or gain.
thinkbiggerdesigns Why Do Atoms Ions
Web both forms represent the same periodic table. An atom is the smallest unit of matter that retains all of the chemical properties of an element. Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and. Atoms with the same number of protons but different numbers of neutrons are called isotopes of the same chemical element..
2.6 Molecules and Molecular Compounds Chemistry LibreTexts
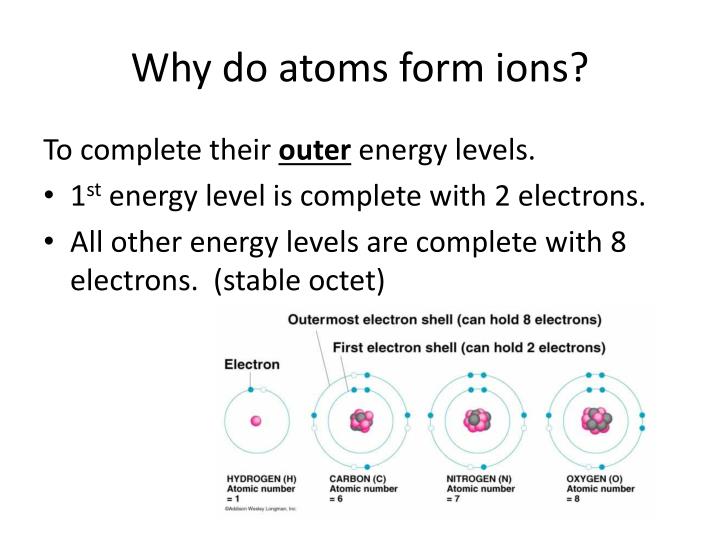
Usually an atom has the same number of electrons as protons. Adding a proton to an atom makes a new element, while adding a. Atoms can lose or gain electrons. They are listed on the periodic table. Web atoms of the same element have the same number of protons, called the atomic number.
What Is an Atom? Live Science
Web home and his team used the shared vibrational motion of two ionized atoms—one calcium and one beryllium—to create their phonon laser. Instead, they’re usually interacting with other atoms (or groups of atoms). Much of the study of chemistry, however, involves looking at what happens when atoms combine with other atoms to form compounds. In chemistry, an element is a.
Periodic Variations in Element Properties Chemistry I
Basically, any material with a composition that includes more than one element symbol or that has a subscript. Web the number of electrons in an atom is always the same as the number of protons, so atoms are electrically neutral. A) a gas b) matter (correct) c) a solid d) organic matter with definite weight and volume but no definite.
Elements and Atoms The Building Blocks of Matter · Anatomy and Physiology
Molecules and compounds consist of atoms but are not themselves atoms.examples of molecules and. The number of neutrons is very roughly the same as the number of protons, but sometimes it's rather more. If a diatomic molecule consists of two atoms of the same element, such as hydrogen (h 2) or oxygen (o 2), then it is said to be.
Scientific Proof of God, A New and Modern Bible, and Coexisting
The number of neutrons is very roughly the same as the number of protons, but sometimes it's rather more. Web as atoms come together to form molecules, chemical bonds bind them together. A) a gas b) matter (correct) c) a solid d) organic matter with definite weight and volume but no definite shape is. Examples of elements are carbon and.
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
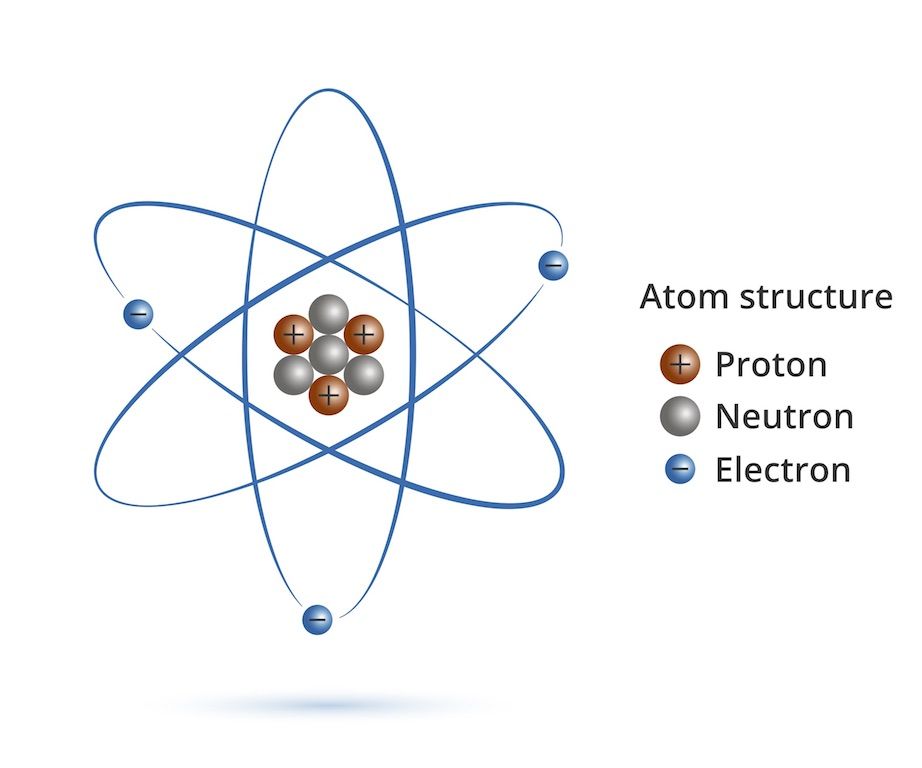
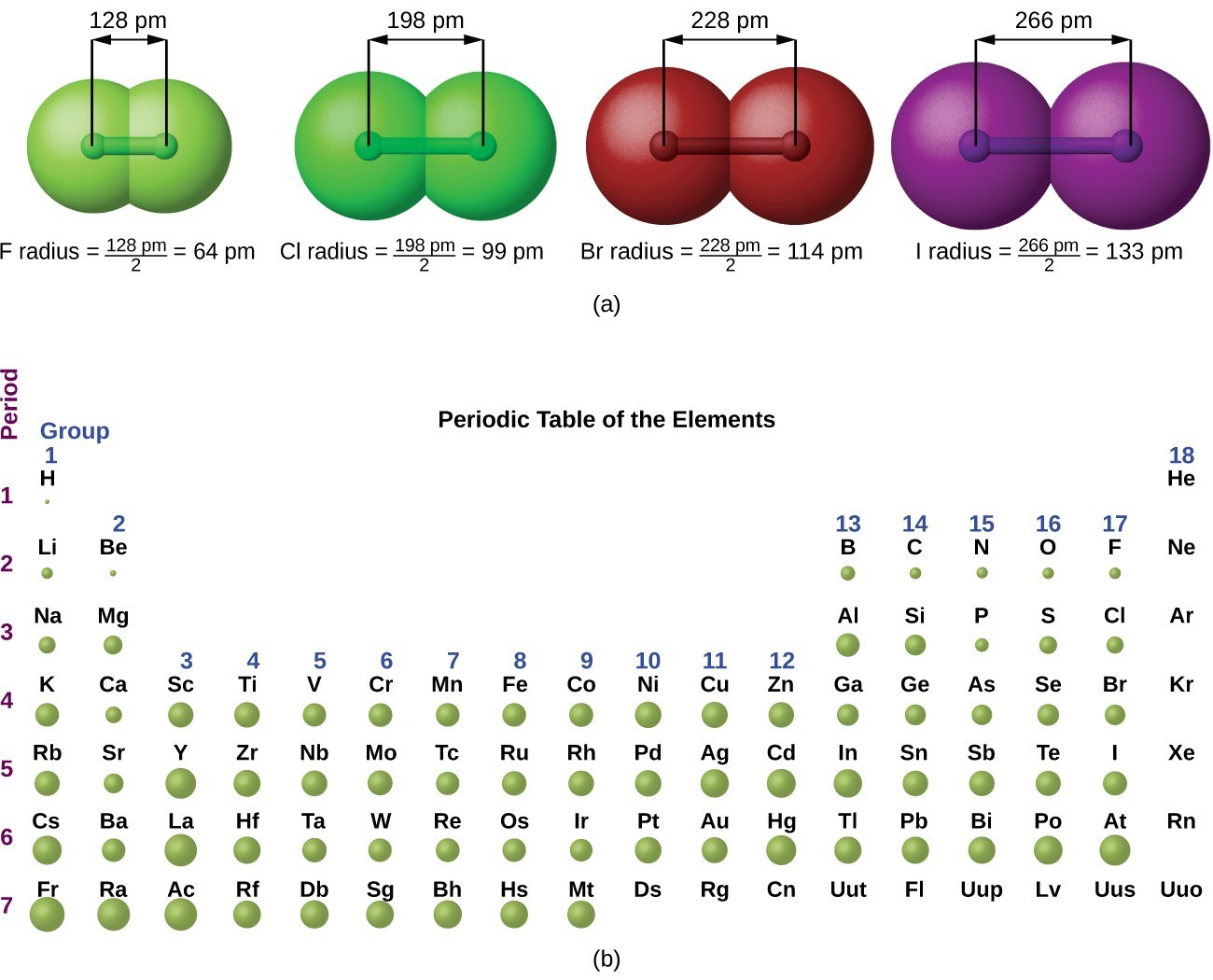
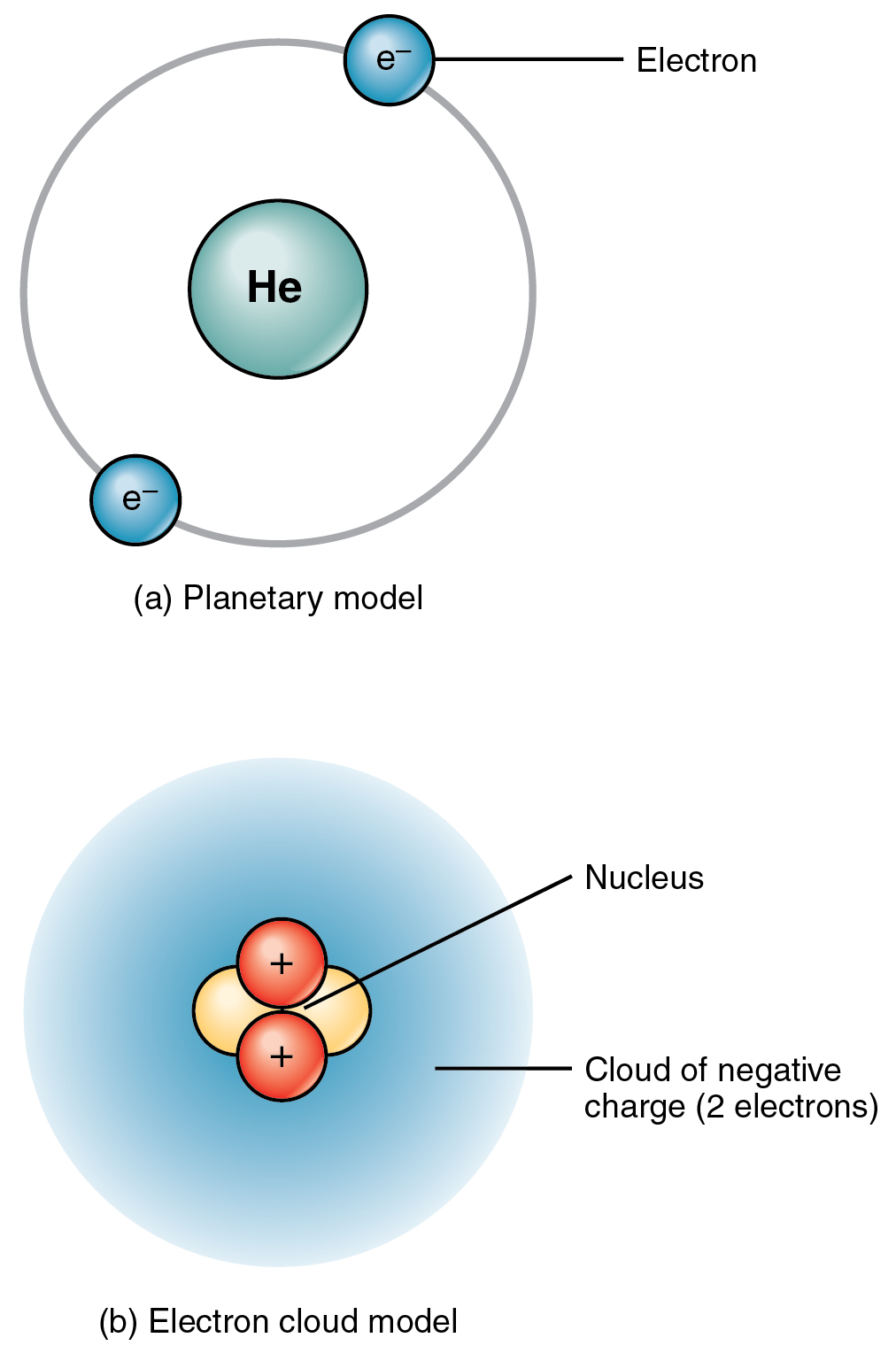
Dalton based his theory on the law of conservation of mass and the law of constant composition. Web in all atoms, the number of protons and the number of electrons is always the same. Usually an atom has the same number of electrons as protons. Web all atoms are roughly the same size, whether they have 3 or 90 electrons..
The Three Atoms Bond Together, Forming A Stable Molecule.
Web because an atom usually has the same number of electrons as protons, the atomic number identifies the usual number of electrons as well. Instead, they’re usually interacting with other atoms (or groups of atoms). Web atoms are the smallest units of matter that still retain the fundamental chemical properties of an element. Ions similarly, atoms of the same element may have more or less electrons, and an atom with a net electric charge (i.e.
Web Atoms Always Have An Equal Number Of Protons And Electrons, And The Number Of Protons And Neutrons Is Usually The Same As Well.
Web introduction only when two atoms of the same element form a covalent bond are the shared electrons actually shared equally between the atoms. So nitrogen has 7 protons and 7 electrons, calcium has 20 protons and 20 electrons, and tin has 50 protons and 50 electrons. In their most common form, many elements also contain the same number of neutrons as protons. Web the atoms in one element are all the same as each other, but they are different from the atoms of any other elements.
Dalton Based His Theory On The Law Of Conservation Of Mass And The Law Of Constant Composition.
Web atoms of the same element have the same number of protons, called the atomic number. Web learn about the structure of the atom, and how atoms make up matter. Atoms with the same number of protons but different numbers of neutrons are called isotopes of the same chemical element. Web introduction living things are made up of atoms, but in most cases, those atoms aren’t just floating around individually.
If A Diatomic Molecule Consists Of Two Atoms Of The Same Element, Such As Hydrogen (H 2) Or Oxygen (O 2), Then It Is Said To Be Homonuclear.
A compound is a distinct group of atoms held together by chemical bonds. Within a single element, the number of neutrons may vary, determining the isotope of that element. But do affect its weight. Each atom of hydrogen has two electrons and the.

![Molecules and Compounds Definition, Differenences [in Table Form]](https://d1avenlh0i1xmr.cloudfront.net/large/756bdbc0-0026-418f-bc9f-de699cc72183/molecules-of-single-element-and-their-atomicity-teachoo-01.jpg)