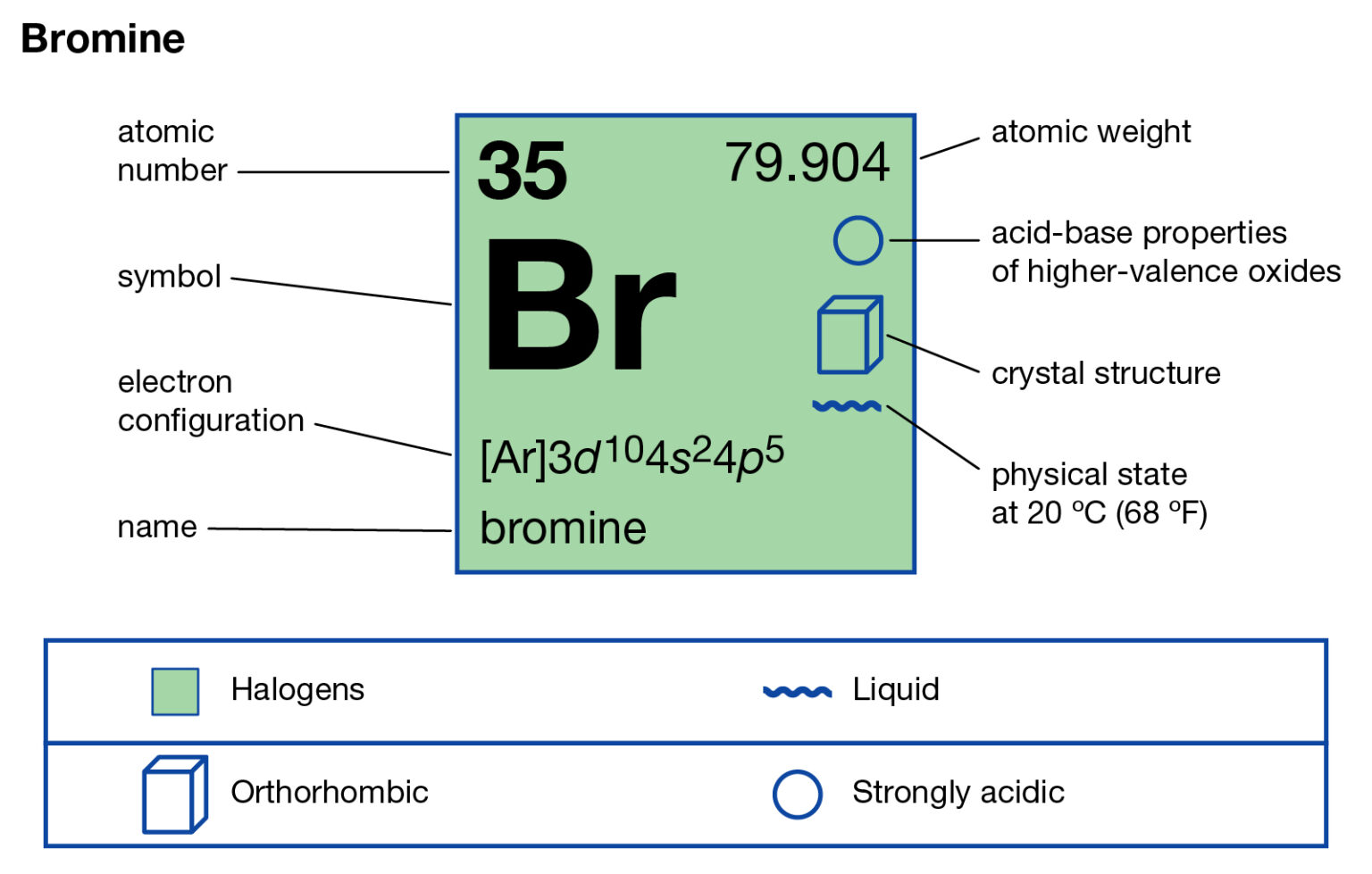
Bromine Electron Configuration Long Form
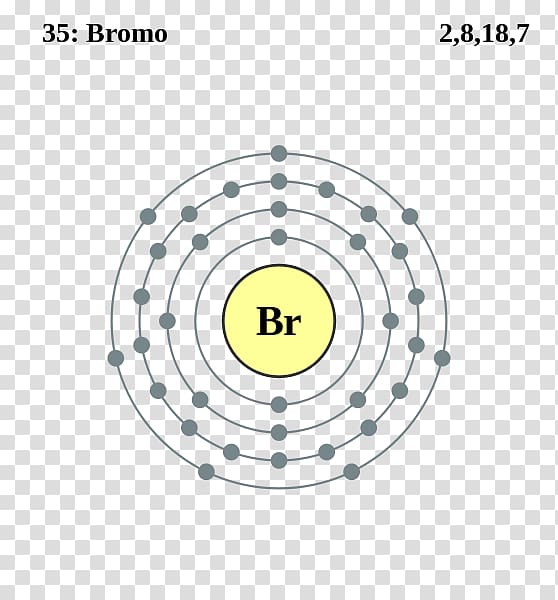
Bromine Electron Configuration Long Form - Web using aufbau principle. Web let’s use it to write the electron configuration of a neutral bromine atom, a bromine atom has 35 electrons. Melting point (br 2) 265.8 k (−7.2 °c, 19 °f) boiling point (br 2) 332.0 k (58.8 °c, 137.8 °f) density (near r.t.) br 2, liquid: Electron configuration of oxygen (o. Web electron configuration of bromine is [ar] 3d10 4s2 4p5. Web the electron configuration of bromine shows that the last shell of bromine has seven electrons. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. The elements of group 17 have seven electrons in their outermost shell. | socratic what is the electron configuration for bromine? 265.90 k, 5.8 kpa :
Density is defined as the mass per unit volume. Some are hard to memorise (or predict), so what is the electron configuration of an atom of br? Write the electron configuration for bromine. Web electron configuration of beryllium (be) [he] 2s 2: 265.90 k, 5.8 kpa : So, the valence electronic configuration of group 17 is n s 2 n p 5. The elements of group 17 have seven electrons in their outermost shell. Web the arrangement of electrons in an atom by a superscript, in each sublevel is known as electron configuration. The atomic number if bromine is 35 thus there are 35 electrons present which fill accorindg to the hund’s rule and aufbau principle a follows: | socratic what is the electron configuration for bromine?
Web electron configuration the arrangements of electrons above the last (closed shell) noble gas. Electron configuration of oxygen (o. In the case of bromine the abbreviated electron configuration is [ar] 3d10 4s2 4p5. Electron configuration of carbon (c) [he] 2s 2 2p 2: Therefore, the valence electrons of bromine are seven. In lewis electron dot diagram of bromine. Density is defined as the mass per unit volume. Electronic configuration of group 17: | socratic what is the electron configuration for bromine? What is the complete electron configuration of bromine?
Lewis Dot Diagram For Bromine Free Wiring Diagram
The elements of group 17 have seven electrons in their outermost shell. This can be shortened to [ar]4s23d104p5. 1s 2 2s 2 2p 2: The complete electron configuration of bromine is 1s22s2 2p63s23p64s2 3d10 4p5. Web electron configuration the arrangements of electrons above the last (closed shell) noble gas.
Orbital Diagram For Rubidium
This can be shortened to [ar]4s23d104p5. Web using aufbau principle. Using the blocks in the periodic table we can write the electron configuration of bromine as: Density of bromine density of bromine is 3.12g/cm3. You may use the core notation or the long form.
Bromine ) on emaze
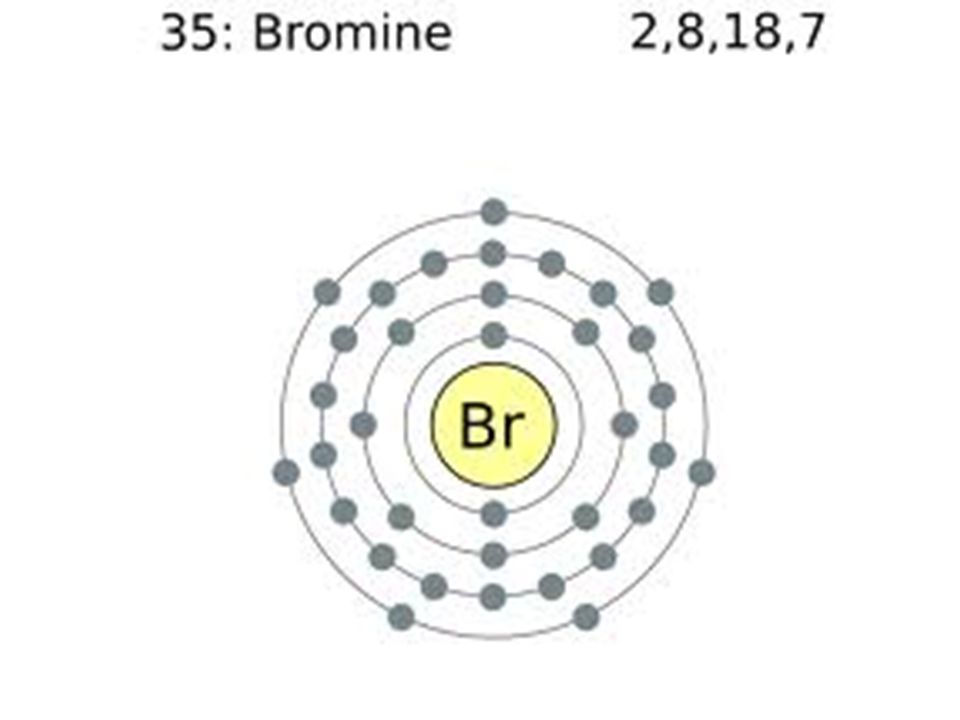
Typical densities of various substances are at atmospheric pressure. You may use the core notation or the long form. Electron configuration of boron (b) [he] 2s 2 2p 1: The elements of group 17 have seven electrons in their outermost shell. Web electrons have a specific form of distribution (or configuration) in every atom, even bromine.
Excited State Electron Configuration of Bromine
Density is defined as the mass per unit volume. 1s 2 2s 2 2p 2: Using the blocks in the periodic table we can write the electron configuration of bromine as: Second, make a table of subshell and its maximum electrons. Please be sure to properly use superscripts and subscripts using the t2 or t2 button.
Electron Configuration of Bromine, Br YouTube
In the case of bromine the abbreviated electron configuration is [ar] 3d10 4s2 4p5. Web let’s use it to write the electron configuration of a neutral bromine atom, a bromine atom has 35 electrons. Web electron configuration the arrangements of electrons above the last (closed shell) noble gas. Web electron configuration 3d 10 4s 2 4p 5: 1s 2 2s.
Bromine Periodic Table Square Periodic Table Timeline
Electronic configuration of group 17: Web electron configuration of beryllium (be) [he] 2s 2: | socratic what is the electron configuration for bromine? You can see that 3p is coming before the 4p and after the 4s. Write the electron configuration for bromine.
Atom Diagrams Electron Configurations of the Elements
Web using aufbau principle. Write the electron configuration for bromine. Using the blocks in the periodic table we can write the electron configuration of bromine as: Web electron configuration of beryllium (be) [he] 2s 2: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5.
Bromine Valence Electrons Bromine Valency (Br) Dot Diagram
Electron configurations of elements beyond hassium (element 108) have never been measured; What is the complete electron configuration of bromine? First, find electrons of bromine atom. Chemical element halogen see all related content → bromine (br), chemical element, a deep red noxious liquid, and a member of the halogen elements, or group 17 (group viia) of the periodic table. The.
How Can We Find A Electron Configuration For Bromine (Br)
In lewis electron dot diagram of bromine. Please be sure to properly use superscripts and subscripts using the t2 or t2 button. This can be shortened to [ar]4s23d104p5. Web electrons have a specific form of distribution (or configuration) in every atom, even bromine. Web there are 35 arrows in the electron configuration for bromine, which is for orbital filling.
Bromine Electron Configuration (Br) with Orbital Diagram
Chemical element halogen see all related content → bromine (br), chemical element, a deep red noxious liquid, and a member of the halogen elements, or group 17 (group viia) of the periodic table. Web there are 35 arrows in the electron configuration for bromine, which is for orbital filling. Bromine has an electron configuration of 1s 2 2s 2 2p.
All You Need To Do Is Work Your Way Across The Periodic Table Filling The Orbitals As You Go
Web using aufbau principle. | socratic what is the electron configuration for bromine? Chemistry electron configuration electron configuration 1 answer zach dec 24, 2015 [ar] 4s23d104p5 explanation: First, find electrons of bromine atom.
Melting Point (Br 2) 265.8 K (−7.2 °C, 19 °F) Boiling Point (Br 2) 332.0 K (58.8 °C, 137.8 °F) Density (Near R.t.) Br 2, Liquid:
Electron configurations of elements beyond hassium (element 108) have never been measured; Web electron configuration the arrangements of electrons above the last (closed shell) noble gas. Web the electron configuration of bromine shows that the last shell of bromine has seven electrons. Write the electron configuration for bromine.
Please Be Sure To Properly Use Superscripts And Subscripts Using The T2 Or T2 Button.
Chemical element halogen see all related content → bromine (br), chemical element, a deep red noxious liquid, and a member of the halogen elements, or group 17 (group viia) of the periodic table. Some are hard to memorise (or predict), so what is the electron configuration of an atom of br? Web there are 35 arrows in the electron configuration for bromine, which is for orbital filling. Since the atomic number of bromine is 35, the total electrons of bromine are 35.
1S 2 2S 2 2P 2:
Web the arrangement of electrons in an atom by a superscript, in each sublevel is known as electron configuration. The complete electron configuration of bromine is 1s22s2 2p63s23p64s2 3d10 4p5. Web this page shows the electron configurations of the neutral gaseous atoms in their ground states. Using the blocks in the periodic table we can write the electron configuration of bromine as:
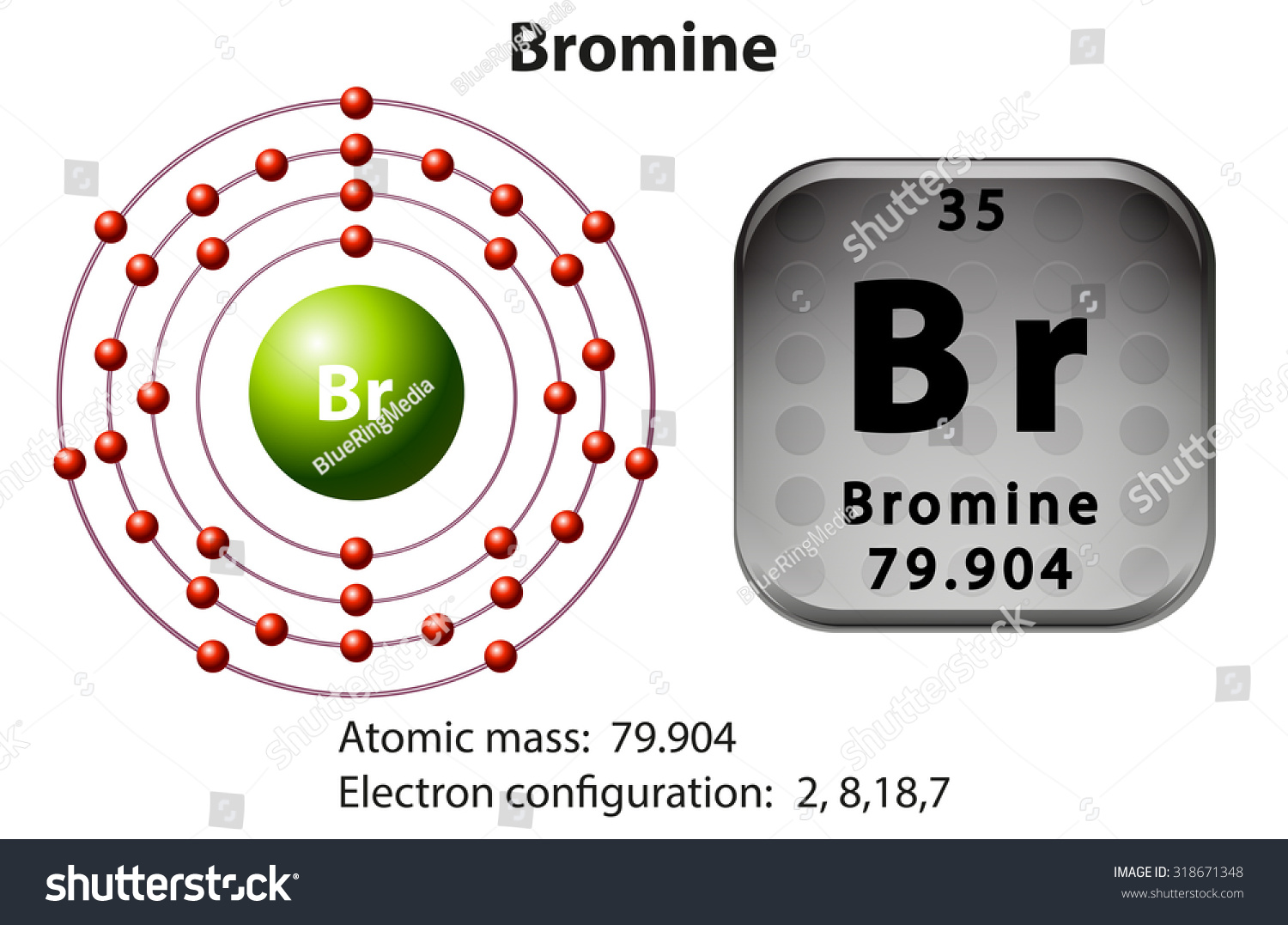

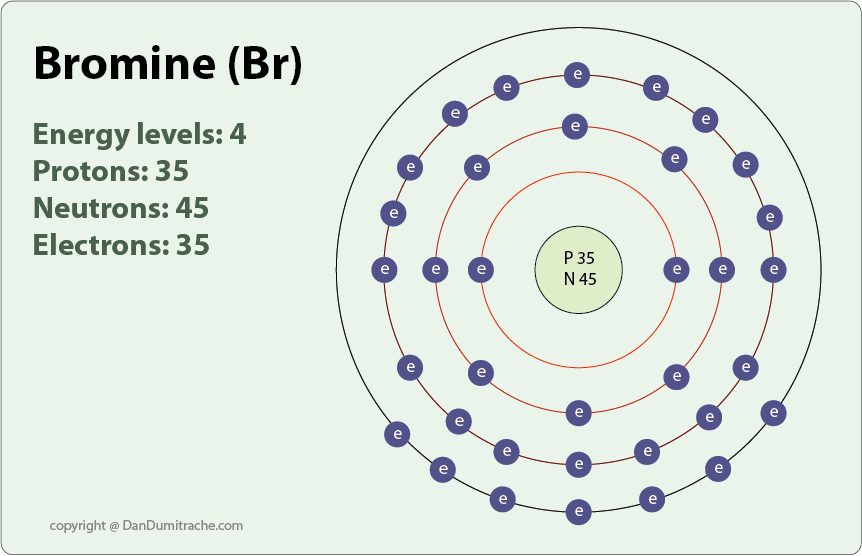

:max_bytes(150000):strip_icc()/Bromine-58b601f93df78cdcd83d2817.jpg)