Can Hcl Form Hydrogen Bonds
Can Hcl Form Hydrogen Bonds - So when you have one ionic bond vs one hydrogen bond, ionic wins,. Web hydrogen can only form 1 bond. The correct option is d. It results from the attractive force between a. Web hydrogen chloride may be formed by the direct combination of chlorine (cl 2) gas and hydrogen (h 2) gas; Making the other hydrogen halides. Between two hcl (intermolecular) their is no hydrogen bond either, because hcl have a greater diameter. Web the molecules of hcl, hbr and hi do not form a hydrogen bond. Web hydrogen bonds form between the δ+ hydrogen on one hf molecule and a lone pair on the fluorine of another one.the figure below illustrates this association: Web small amounts of hydrogen chloride for laboratory use can be generated in an hcl generator by dehydrating hydrochloric acid with either sulfuric acid or anhydrous.
Web hydrogen can only form 1 bond. Web so, hydrogen bonding is the electrostatic force of attraction between partial positive hydrogen atom and a highly electronegative atom like f, o and n belonging to another. Web small amounts of hydrogen chloride for laboratory use can be generated in an hcl generator by dehydrating hydrochloric acid with either sulfuric acid or anhydrous. Making the other hydrogen halides. It has a polar covalent bond (intramolecular forces). Web hydrogen bonds form between the δ+ hydrogen on one hf molecule and a lone pair on the fluorine of another one.the figure below illustrates this association: Web hydrogen chloride may be formed by the direct combination of chlorine (cl 2) gas and hydrogen (h 2) gas; The hydrogen atom will share its 1 electron with chlorine to form one covalent bond and make a hydrogen chloride molecule (hcl). H2o can form hydrogen bonds. The reaction is rapid at temperatures above 250 °c (482.
So when you have one ionic bond vs one hydrogen bond, ionic wins,. All of the hydrogen halides can be made in an exactly similar way using concentrated. Web is hcl a hydrogen bond? Web the accumulation of several partially polar h2o molecules overpowers the singular charge of the ionic bond. The reaction is rapid at temperatures above 250 °c (482. Web hydrogen chloride may be formed by the direct combination of chlorine (cl 2) gas and hydrogen (h 2) gas; Web hydrogen bonds form between the δ+ hydrogen on one hf molecule and a lone pair on the fluorine of another one.the figure below illustrates this association: Web chlorine is a large atom, making the hcl have a high electronegativity measure, which is not significant enough to create a hydrogen bond. H2o can form hydrogen bonds. It has a polar covalent bond (intramolecular forces).
What is a hydrogen bond? [With free chemistry study guide]
Web chlorine is a large atom, making the hcl have a high electronegativity measure, which is not significant enough to create a hydrogen bond. Between two hcl (intermolecular) their is no hydrogen bond either, because hcl have a greater diameter. Web hydrogen can only form 1 bond. Web hydrogen chloride may be formed by the direct combination of chlorine (cl.
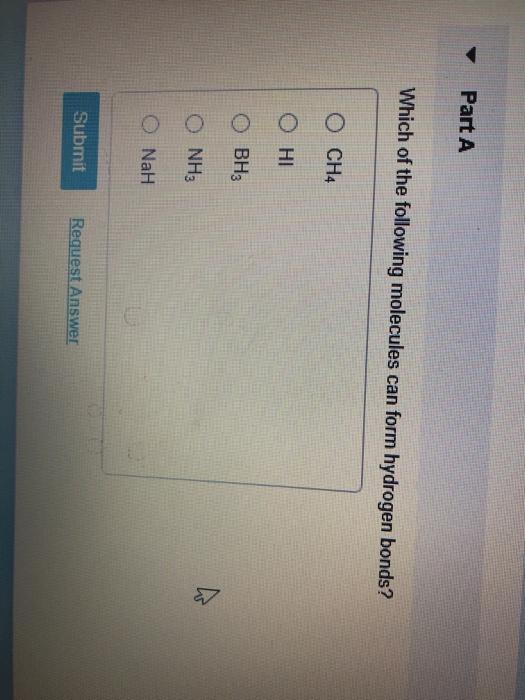
Solved Part A Which of the following molecules can form
So when you have one ionic bond vs one hydrogen bond, ionic wins,. Web small amounts of hydrogen chloride for laboratory use can be generated in an hcl generator by dehydrating hydrochloric acid with either sulfuric acid or anhydrous. The reaction is rapid at temperatures above 250 °c (482. Web hydrogen can only form 1 bond. The correct option is.
BioSynthesis Chapter 2 The Chemical Basis of Life I Atoms, Molecules
It results from the attractive force between a. So when you have one ionic bond vs one hydrogen bond, ionic wins,. Web is hcl a hydrogen bond? H2o can form hydrogen bonds. Web hydrogen chloride may be formed by the direct combination of chlorine (cl 2) gas and hydrogen (h 2) gas;
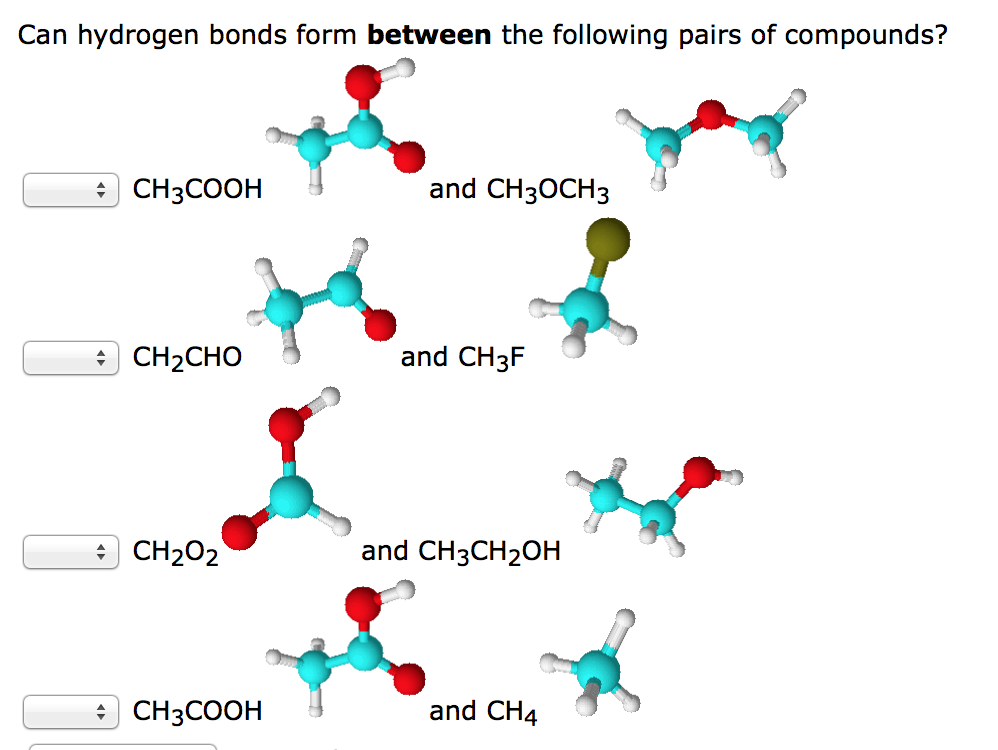
Solved Can hydrogen bonds form between the following pairs
Solution hcl is a covalent bond because when atoms with different electronegativity exchange electrons in a covalent link to form a polar covalent. Web hydrogen can only form 1 bond. The reaction is rapid at temperatures above 250 °c (482. The hydrogen atom will share its 1 electron with chlorine to form one covalent bond and make a hydrogen chloride.
Properties of Water Presentation Biology
Web is hcl a hydrogen bond? All of the hydrogen halides can be made in an exactly similar way using concentrated. Solution hcl is a covalent bond because when atoms with different electronegativity exchange electrons in a covalent link to form a polar covalent. Making the other hydrogen halides. Web hydrogen bonds form between the δ+ hydrogen on one hf.
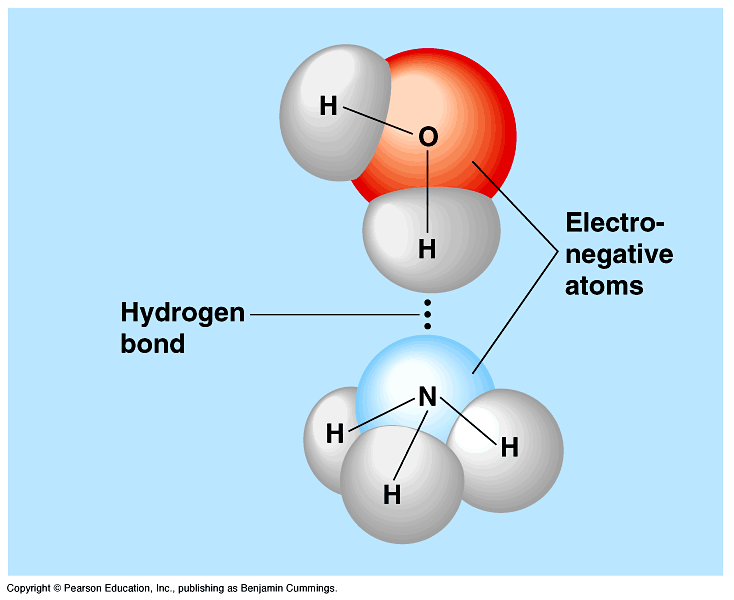
Why do H2O Molecules form more Hydrogen Bonds compared to NH3 and HF
Web the accumulation of several partially polar h2o molecules overpowers the singular charge of the ionic bond. The reaction is rapid at temperatures above 250 °c (482. Web small amounts of hydrogen chloride for laboratory use can be generated in an hcl generator by dehydrating hydrochloric acid with either sulfuric acid or anhydrous. Between two hcl (intermolecular) their is no.
Hydrogen bonds YouTube
Web small amounts of hydrogen chloride for laboratory use can be generated in an hcl generator by dehydrating hydrochloric acid with either sulfuric acid or anhydrous. H2o can form hydrogen bonds. It has a polar covalent bond (intramolecular forces). Web hydrogen chloride may be formed by the direct combination of chlorine (cl 2) gas and hydrogen (h 2) gas; The.
Bonds That Hold Water Molecules Together / Intermolecular Forces
H2o can form hydrogen bonds. The hydrogen atom will share its 1 electron with chlorine to form one covalent bond and make a hydrogen chloride molecule (hcl). Web hydrogen can only form 1 bond. Web is hcl a hydrogen bond? Web hydrogen bonds form between the δ+ hydrogen on one hf molecule and a lone pair on the fluorine of.
Can Carbon Form bonds without Hybridization? Chemistry Stack Exchange
Making the other hydrogen halides. So when you have one ionic bond vs one hydrogen bond, ionic wins,. H2o can form hydrogen bonds. All of the hydrogen halides can be made in an exactly similar way using concentrated. The reaction is rapid at temperatures above 250 °c (482.
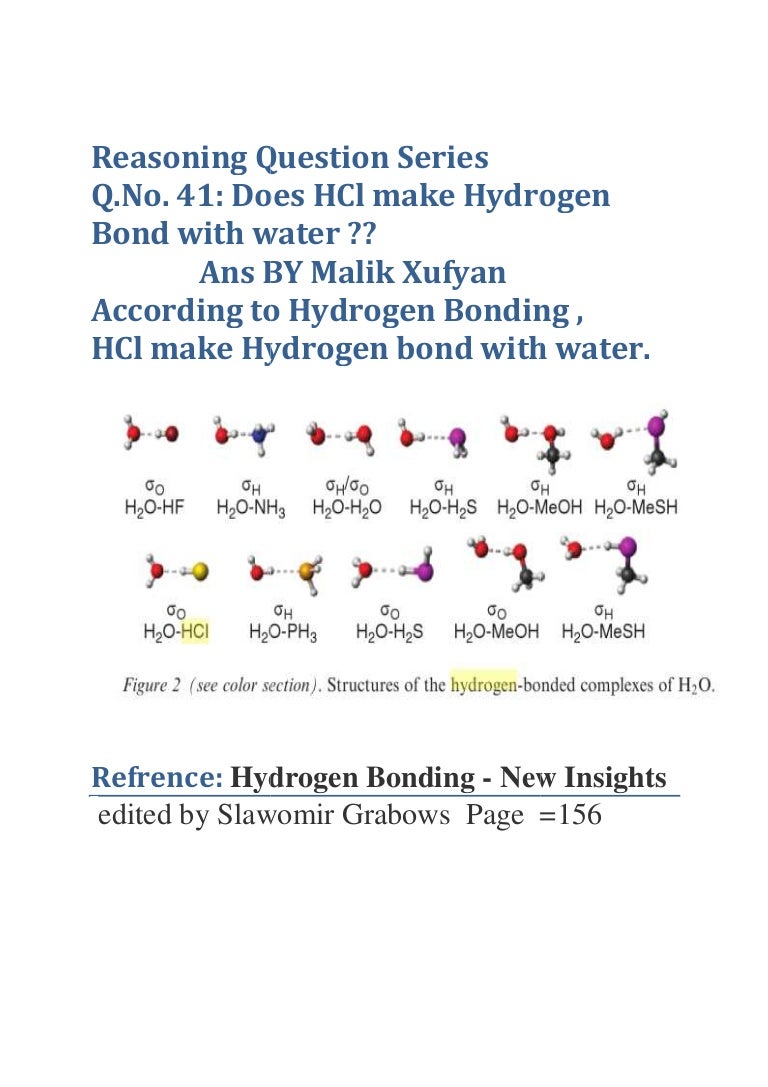
Q.No. 41 Does HCl make Hydrogen Bond with water ?? Malik Xufyan
Making the other hydrogen halides. It results from the attractive force between a. Web is hcl a hydrogen bond? Web small amounts of hydrogen chloride for laboratory use can be generated in an hcl generator by dehydrating hydrochloric acid with either sulfuric acid or anhydrous. Web hydrogen bonds form between the δ+ hydrogen on one hf molecule and a lone.
Web Chlorine Is A Large Atom, Making The Hcl Have A High Electronegativity Measure, Which Is Not Significant Enough To Create A Hydrogen Bond.
The reaction is rapid at temperatures above 250 °c (482. Web the molecules of hcl, hbr and hi do not form a hydrogen bond. The correct option is d. All of the hydrogen halides can be made in an exactly similar way using concentrated.
Making The Other Hydrogen Halides.
It results from the attractive force between a. Web hydrogen chloride may be formed by the direct combination of chlorine (cl 2) gas and hydrogen (h 2) gas; H2o can form hydrogen bonds. The hydrogen atom will share its 1 electron with chlorine to form one covalent bond and make a hydrogen chloride molecule (hcl).
It Has A Polar Covalent Bond (Intramolecular Forces).
Web is hcl a hydrogen bond? Web hydrogen bonds form between the δ+ hydrogen on one hf molecule and a lone pair on the fluorine of another one.the figure below illustrates this association: Web nacl + h 3 po 4 hcl + nah 2 po 4. Web small amounts of hydrogen chloride for laboratory use can be generated in an hcl generator by dehydrating hydrochloric acid with either sulfuric acid or anhydrous.
Web The Accumulation Of Several Partially Polar H2O Molecules Overpowers The Singular Charge Of The Ionic Bond.
Web hydrogen can only form 1 bond. Solution hcl is a covalent bond because when atoms with different electronegativity exchange electrons in a covalent link to form a polar covalent. So when you have one ionic bond vs one hydrogen bond, ionic wins,. Web so, hydrogen bonding is the electrostatic force of attraction between partial positive hydrogen atom and a highly electronegative atom like f, o and n belonging to another.
![What is a hydrogen bond? [With free chemistry study guide]](http://www.aceorganicchem.com/blog/wp-content/uploads/2018/04/4-22-2018-6-20-10-PM.jpg)
.PNG)