Chapter 8 Review Chemistry
Chapter 8 Review Chemistry - Click the card to flip 👆. Unit 11 states of matter and intermolecular forces. Single bonds have a bond order of one, and multiple bonds with bond orders of two (a double bond) and three (a. Web 4.4 (5 reviews) tell the contributions of döbereiner to the periodic table. Smallest particle of an element; Molecular compounds have relatively high boiling points. Web chemistry chapter 8 review 4.3 (3 reviews) get a hint explain why elements such as p, n, and s have positive oxidation numbers in some compounds but have negative oxidation numbers in others? Why is it important for an equation to be balanced? Both types of bonds result from overlap of atomic orbitals on adjacent atoms and contain a maximum of two electrons. The following pages contain the bulk (but not all) of the information for the chapter.
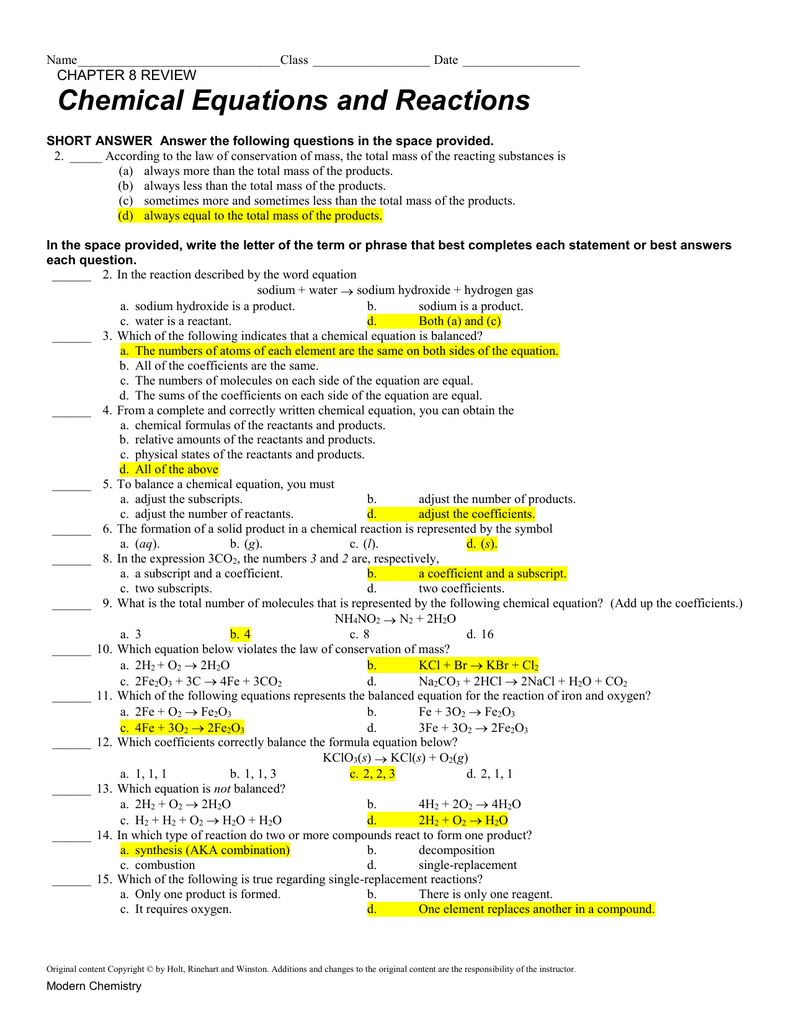
Write the balanced chemical equation for the combustion of c2h2 in oxygen. Web chapter 8 chemistry review the state of matter (phase) for a reactant or a product in a chemical equation is indicated by a. Web review of important concepts. Web holt modern chemistry review. Web terms in this set (44) a diatomic molecule contains two or three atoms. Strengths of covalent bonds bond order is the number of electron pairs that hold two atoms together. Unit 11 states of matter and intermolecular forces. Web 4.4 (5 reviews) tell the contributions of döbereiner to the periodic table. Molecular compounds have relatively high boiling points. Is the common name for ethyne, used
Web 4.4 (5 reviews) tell the contributions of döbereiner to the periodic table. Web holt modern chemistry review. They form crystal lattices because their positive ions and negative ions are so tightly attracted. Web we go over hydroboration, markovnikov additions, kmno4 and oso4 reactions, and a ton of other reaction mechanisms. Acetylene gas, c2h2, is burned to provide the high temperature needed in welding. Web modern chemistry 1 chemical equations and reactions chapter 8 review chemical equations and reactions teacher notes and answers chapter 8 section 1 short answer 1. The following pages contain the bulk (but not all) of the information for the chapter. Unit 10 gases and kinetic molecular theory. Click the card to flip 👆 symbol in parentheses after the formula click the card to flip 👆 1 / 23. Web terms in this set (44) a diatomic molecule contains two or three atoms.
Spice of Lyfe Chemical Equations And Reactions Chapter 8 Review Answers
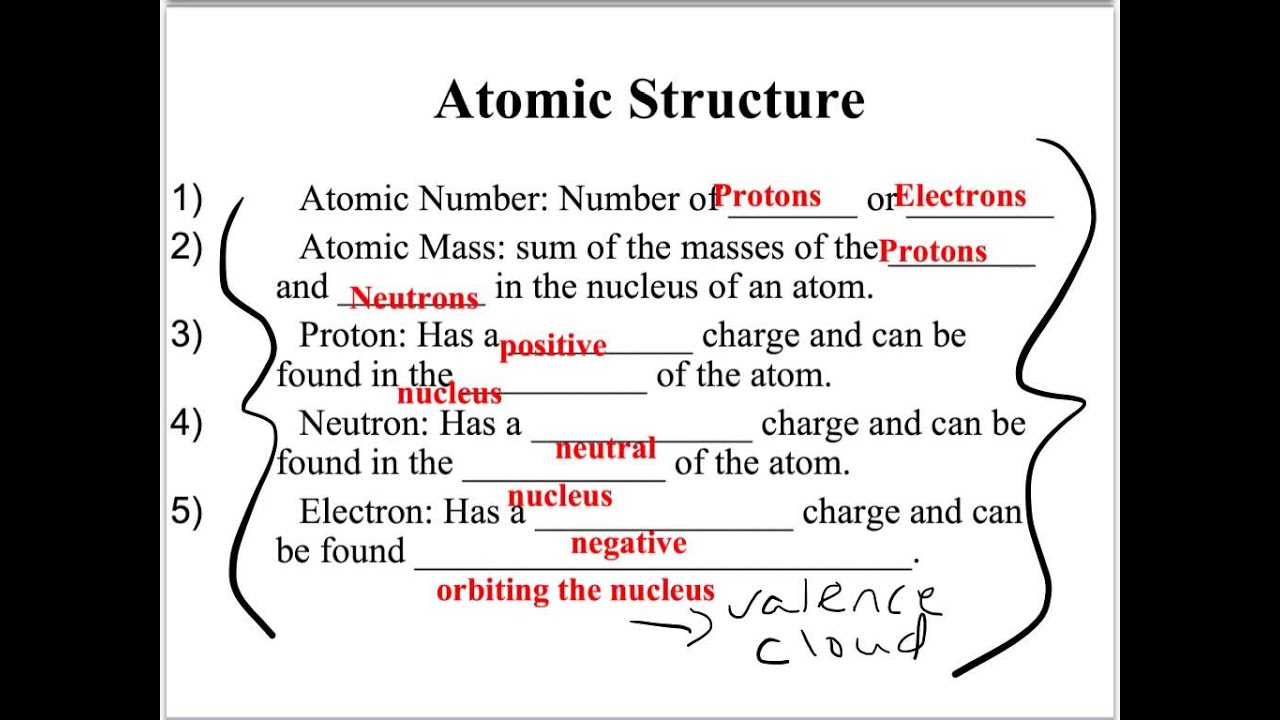
Contains protons, neutrons, and electrons. Web unit 6 more about chemical reactions. Smallest particle of an element; Molecular compounds have relatively high boiling points. Unit 10 gases and kinetic molecular theory.
Chemistry Final Review YouTube
Unit 11 states of matter and intermolecular forces. 1.match the symbol on the left with its appropriate description on the right. The following pages contain the bulk (but not all) of the information for the chapter. Web modern chemistry 1 chemical equations and reactions chapter 8 review chemical equations and reactions teacher notes and answers chapter 8 section 1 short.
Spice of Lyfe Chemical Equations And Reactions Chapter 8 Review Answers
Web unit 6 more about chemical reactions. They form crystal lattices because their positive ions and negative ions are so tightly attracted. Web bju chemistry chapter 8 review 2.8 (4 reviews) oxidation number (on) click the card to flip 👆 the number of electrons (the charge) that an atom in a compound must gain or lose to return to its.
Unit 8 Review General Chemistry
Strengths of covalent bonds bond order is the number of electron pairs that hold two atoms together. Acetylene gas, c2h2, is burned to provide the high temperature needed in welding. Web terms in this set (27) atom. What is an ionic compound whose solution conducts an electric current? Unit 10 gases and kinetic molecular theory.
CBSE 12th Chemistry Board Exam 2020 Important Questions & Answers from
They form crystal lattices because their positive ions and negative ions are so tightly attracted. Web terms in this set (20) why do ionic compounds form crystal lattices? Strengths of covalent bonds bond order is the number of electron pairs that hold two atoms together. Click the card to flip 👆. Web 4.4 (5 reviews) tell the contributions of döbereiner.
AP Chemistry Chapter 8 & 9 Practice Test with answers (1)
Web unit 6 more about chemical reactions. Click the card to flip 👆. Both types of bonds result from overlap of atomic orbitals on adjacent atoms and contain a maximum of two electrons. Strengths of covalent bonds bond order is the number of electron pairs that hold two atoms together. Web terms in this set (27) atom.
Chemistry Chapter 8 Review Problems YouTube
Web modern chemistry 1 chemical equations and reactions chapter 8 review chemical equations and reactions teacher notes and answers chapter 8 section 1 short answer 1. Web bju chemistry chapter 8 review 2.8 (4 reviews) oxidation number (on) click the card to flip 👆 the number of electrons (the charge) that an atom in a compound must gain or lose.
Selina Concise Chemistry Class 8 ICSE Solutions Chapter 1 Matter
Web terms in this set (27) atom. Acetylene gas, c2h2, is burned to provide the high temperature needed in welding. Web holt modern chemistry review. Is the common name for ethyne, used Web bju chemistry chapter 8 review 2.8 (4 reviews) oxidation number (on) click the card to flip 👆 the number of electrons (the charge) that an atom in.
Fantastic Chapter 8 Chemistry Test Answers Chemical Equations And
Web chapter 8 chemistry review the state of matter (phase) for a reactant or a product in a chemical equation is indicated by a. Web bju chemistry chapter 8 review 2.8 (4 reviews) oxidation number (on) click the card to flip 👆 the number of electrons (the charge) that an atom in a compound must gain or lose to return.
Chapter 8 Practice Test Doral Academy Preparatory
Web bju chemistry chapter 8 review 2.8 (4 reviews) oxidation number (on) click the card to flip 👆 the number of electrons (the charge) that an atom in a compound must gain or lose to return to its neutral state;. Unit 11 states of matter and intermolecular forces. What does it mean to say an equation is balanced? Web chapter.
Web Review Of Important Concepts.
Single bonds have a bond order of one, and multiple bonds with bond orders of two (a double bond) and three (a. Why is it important for an equation to be balanced? Molecular compounds have relatively high boiling points. Unit 10 gases and kinetic molecular theory.
Acetylene Gas, C2H2, Is Burned To Provide The High Temperature Needed In Welding.
Strengths of covalent bonds bond order is the number of electron pairs that hold two atoms together. Contains protons, neutrons, and electrons. Web chapter 8 review chemical equations and reactions section 1 short answer answer the following questions in the space provided. Smallest particle of an element;
The Following Pages Contain The Bulk (But Not All) Of The Information For The Chapter.
An introduction to organic synthesis alkynes contain triple bond acetylene: Web modern chemistry 1 chemical equations and reactions chapter 8 review chemical equations and reactions teacher notes and answers chapter 8 section 1 short answer 1. Unit 7 electronic structure of atoms. Web terms in this set (27) atom.
Click The Card To Flip 👆 Döbereiner One Of The Ealiest Chemists To Show Similarity Of Properties For Groups Of Elements By Arranging Them In.
1.match the symbol on the left with its appropriate description on the right. Unit 11 states of matter and intermolecular forces. Web we go over hydroboration, markovnikov additions, kmno4 and oso4 reactions, and a ton of other reaction mechanisms. What does it mean to say an equation is balanced?