Chem Chapter 7
Chem Chapter 7 - Web the scientific principle which explains the observation that the amount of heat transfer accompanying a change in one direction is numerically equal but opposite in sign to the amount of heat transfer in the opposite direction is the law of conservation of energy. The protons in the nucleus do not change during normal chemical reactions. C) writing and balancing chemical equations 5. Web chemical reactions are classified into types to help us analyze them and also to help us predict what the products of the reaction will be. Web 1 / 66 flashcards learn test match created by maya_sullivan terms in this set (66) cation a positively charged ion ion an atom that gains or loses electrons anion any atom or group of atoms with a negative charge. N 2h 4(l) + ch 4o(l) → ch 2o(g) + n ∆h = ? Positive charges form when electrons are lost. Web chapter 7 quantum theory and atomic structure. Web seventh grade, chemistry lesson plans. Analysis of the hydrosphere is shared.
P, i, cl, and o would form. Positive charges form when electrons are lost. Our solutions are written by chegg experts so you can be assured of the highest quality! P, i, cl, and o would form. Chapter 7 column click the card to flip 👆 typically, elements share common characteristics with neighbors that occupy the same ______________ of the periodic table. Web seventh grade, chemistry lesson plans. Web seventh grade (grade 7) chemistry questions. Web 1 / 66 flashcards learn test match created by maya_sullivan terms in this set (66) cation a positively charged ion ion an atom that gains or loses electrons anion any atom or group of atoms with a negative charge. Web access chem 11th edition chapter 7 solutions now. Types of chemical reactions and solution stoichiometry 5:
(7 results) an experienced chemistry professor used to say that it took about one explosion per week to maintain college students' attention in chemistry lectures. Only the outer electrons move. Web the scientific principle which explains the observation that the amount of heat transfer accompanying a change in one direction is numerically equal but opposite in sign to the amount of heat transfer in the opposite direction is the law of conservation of energy. Atoms, molecules, and ions 3: P, i, cl, and o would form. Web feb 24, 2020 7.e: Positive charges form when electrons are lost. Web seventh grade, chemistry science projects. Modification of work by nasa) chapter outline 7… The protons in the nucleus do not change during normal chemical reactions.
AP Chem Chapter 17 part 4 YouTube
P, i, cl, and o would form. N 2h 4(l) + ch 4o(l) → ch 2o(g) + n ∆h = ? Select one or more questions using the checkboxes above each. (7 results) an experienced chemistry professor used to say that it took about one explosion per week to maintain college students' attention in chemistry lectures. Web chapter 7 quantum.
Polymer Chem Chapter 7 Dr. Anthony Revis YouTube
Atoms, molecules, and ions 3: Web describe various types of solutions. Some of the worksheets for this concept are term 2 grade 7 natural science work, 7 the chemistry science orbit, science grade 7 chemistry in our world, chemistry computing formula mass work, ck 12 chemistry workbook, mole calculation work, ks3 chemistry. Web chemistry chapter 7 valence electrons click the.
chem 215 chapter 12 YouTube
N2h4(l) + h2(g) → 2nh3(g) reaction 2: Some of the worksheets for this concept are term 2 grade 7 natural science work, 7 the chemistry science orbit, science grade 7 chemistry in our world, chemistry computing formula mass work, ck 12 chemistry workbook, mole calculation work, ks3 chemistry. Web seventh grade (grade 7) chemistry questions. Positive charges form when electrons.
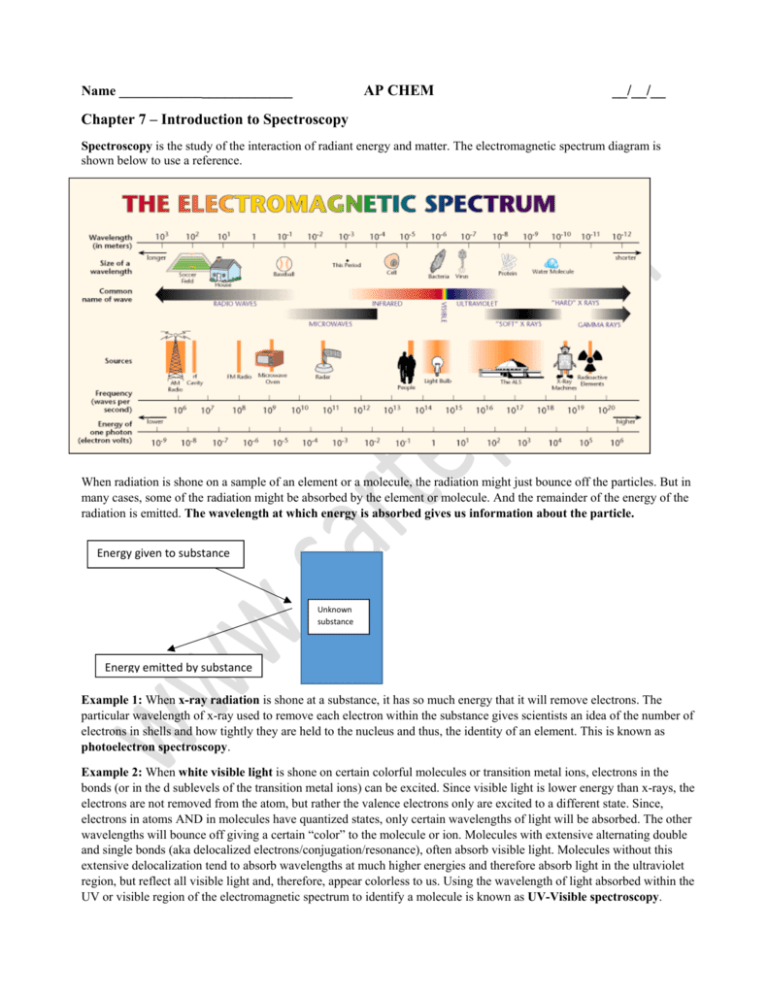
AP CHEM __/__/__ Chapter 7 Introduction to Spectroscopy
C) writing and balancing chemical equations 5. Web seventh grade, chemistry lesson plans. Only the outer electrons move. The protons in the nucleus do not change during normal chemical reactions. (33 results) an experienced chemistry professor used to say that it took about one explosion per week to maintain college students' attention in chemistry lectures.
CHEM 111 Chapter 2 YouTube
Only the outer electrons move. This number helps to determine the chemical. Web chemistry chapter 7 valence electrons click the card to flip 👆 as the groups all have similar chemical properties, this is because of their number of valence electrons. Web the scientific principle which explains the observation that the amount of heat transfer accompanying a change in one.
Gen Chem 2 Chapter 19 Part 1 YouTube
Web 1 / 66 flashcards learn test match created by maya_sullivan terms in this set (66) cation a positively charged ion ion an atom that gains or loses electrons anion any atom or group of atoms with a negative charge. The major component of the solution is called solvent, and the minor component (s) are called solute. This number helps.
Chem 100 Chapter 7 Video 7 YouTube
The five major types of chemical reactions are synthesis, decomposition, single replacement, double replacement, and combustion. P, i, cl, and o would form. Their location determines the reactivity of the atom. If both components in a solution are 50%, the term solute. C) writing and balancing chemical equations 5.
Chem 1 Chapter 7 Lecture Part 1 RJO YouTube
Click the card to flip 👆 1 / 69. The protons in the nucleus do not change during normal chemical reactions. Modification of work by nasa) chapter outline 7… Measurements and analysis in the atmosphere. If both components in a solution are 50%, the term solute.
Chem Chapter 7 Holt 2012
Only the outer electrons move. Web chemistry chapter 7 valence electrons click the card to flip 👆 as the groups all have similar chemical properties, this is because of their number of valence electrons. This number helps to determine the chemical. Web seventh grade, chemistry science projects. The protons in the nucleus do not change during normal chemical reactions.
10th Chem Chapter 3 Lecture 16 Organic Chemistry YouTube
Click the card to flip 👆 1 / 69. Modification of work by nasa) chapter outline 7… Web chemical reactions are classified into types to help us analyze them and also to help us predict what the products of the reaction will be. Web seventh grade, chemistry science projects. P, i, cl, and o would form.
Only The Outer Electrons Move.
You can create printable tests and worksheets from these grade 7 chemistry questions! Chapter 7 column click the card to flip 👆 typically, elements share common characteristics with neighbors that occupy the same ______________ of the periodic table. Some of the worksheets for this concept are term 2 grade 7 natural science work, 7 the chemistry science orbit, science grade 7 chemistry in our world, chemistry computing formula mass work, ck 12 chemistry workbook, mole calculation work, ks3 chemistry. P, i, cl, and o would form.
The Major Component Of The Solution Is Called Solvent, And The Minor Component (S) Are Called Solute.
Measurements and analysis in the atmosphere. Web access chem 11th edition chapter 7 solutions now. Web seventh grade, chemistry lesson plans. Their location determines the reactivity of the atom.
Web Seventh Grade, Chemistry Science Projects.
(7 results) an experienced chemistry professor used to say that it took about one explosion per week to maintain college students' attention in chemistry lectures. The protons in the nucleus do not change during normal chemical reactions. Web the scientific principle which explains the observation that the amount of heat transfer accompanying a change in one direction is numerically equal but opposite in sign to the amount of heat transfer in the opposite direction is the law of conservation of energy. Select one or more questions using the checkboxes above each.
N2H4(L) + H2(G) → 2Nh3(G) Reaction 2:
Only the outer electrons move. Our solutions are written by chegg experts so you can be assured of the highest quality! Types of chemical reactions and solution stoichiometry 5: If both components in a solution are 50%, the term solute.