Cosentyx Enrollment Form 2022 Pdf
Cosentyx Enrollment Form 2022 Pdf - Send out signed cosentyx enrollment form 2022 pdf or print it. Make the steps below to complete cosentyx enrollment form 2022 online easily and. Learn more about cosentyx® & how to get your patients started today. Browse for the cosentyx enrollment form 2023. If a serious infection develops, discontinue cosentyx until the. Please follow your state’s prescribing guidelines for electronic prescriptions (if applicable). Talk to your doctor about submitting an appeal to the. Cosentyx is indicated for the treatment. Ad learn how cosentyx treats multiple conditions & see if it is right for you. Web cosentyx is indicated for the treatment of adult patients with active ankylosing spondylitis.
Program provides initial 5 weekly doses (if prescribed) and monthly doses for free to patients for. Web request form for cosentyx, and be experiencing a delay in obtaining coverage. Ad learn how cosentyx treats multiple conditions & see if it is right for you. Please follow your state’s prescribing guidelines for electronic prescriptions (if applicable). Learn more about cosentyx® & how to get your patients started today. Ad view prescribing, safety & efficacy information for hcps on cosentyx®. Beovu ® 1 888 612 3688. Cosentyx is indicated for the treatment. Web xpose® program enrollment and consent form: Cosentyx ® 1 844 267 3689.
Sign up now for access to a full range of services and support that are. Send out signed cosentyx enrollment form 2022 pdf or print it. Learn more about cosentyx® & how to get your patients started today. Ask about cosentyx & see if it's right for you. If a serious infection develops, discontinue cosentyx until the. Mayzent ® 1 877 629 9368. Cosentyx is indicated for the treatment. Web cosentyx income requirements to be eligible for assistance from the novartis patient assistance foundation (npaf), you must meet the income guidelines,. Download the npaf application form english (pdf 0.1 mb) | spanish (pdf 0.1 mb) Web up to $40 cash back cosentyx srf (successor registration form) is a form that is required to be submitted for patients who have switched from the innovator product to a biosimilar.
COSENTYX 300mg injections 19 & 20 YouTube
Omnitrope ® 1 877 456 6794. Web cosentyx is indicated for the treatment of adult patients with active ankylosing spondylitis. Cosentyx is indicated for the treatment. Make the steps below to complete cosentyx enrollment form 2022 online easily and. Web when considering the use of cosentyx in patients with a chronic infection or a history of recurrent infection.
Cosentyx Sensoready® Pen COSENTYX® (secukinumab)
Learn more about cosentyx® & how to get your patients started today. Web enroll now just considering cosentyx? Web novartis patient support contacts. Send out signed cosentyx enrollment form 2022 pdf or print it. Cosentyx ® 1 844 267 3689.
Program enrollment and consent form XPOSE (English) Intrahealth
Web request form for cosentyx, and be experiencing a delay in obtaining coverage. Web cosentyx is indicated for the treatment of adult patients with active ankylosing spondylitis. Ad learn how cosentyx treats multiple conditions & see if it is right for you. Ask about cosentyx & see if it's right for you. Learn more about cosentyx® & how to get.
Fill Free fillable Drug Prior Authorization Form Cosentyx
Ad learn how cosentyx treats multiple conditions & see if it is right for you. Program provides initial 5 weekly doses (if prescribed) and monthly doses for free to patients for. Web request form for cosentyx, and be experiencing a delay in obtaining coverage. Customize and esign cosentyx start form. Ad view prescribing, safety & efficacy information for hcps on.
Access COSENTYX® (secukinumab)
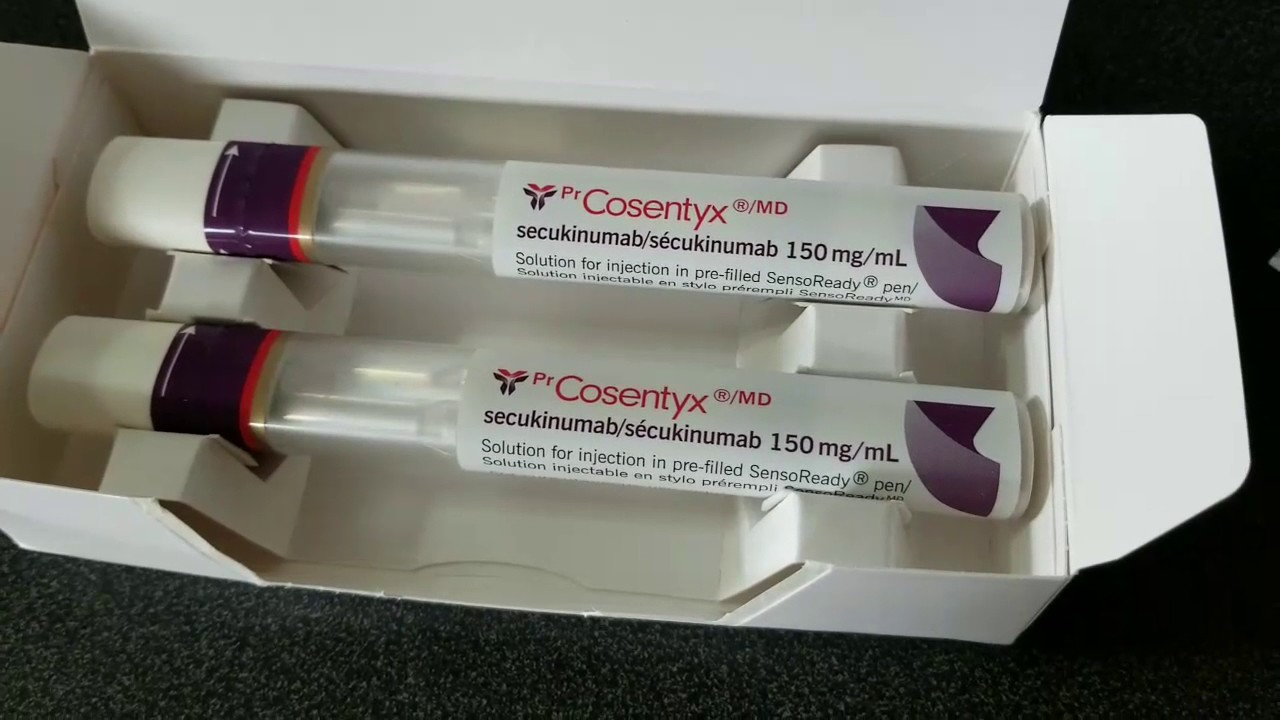
Learn more about cosentyx® & how to get your patients started today. Web when considering the use of cosentyx in patients with a chronic infection or a history of recurrent infection. Ad view prescribing, safety & efficacy information for hcps on cosentyx®. Learn more about cosentyx® & how to get your patients started today. Web cosentyx 150 mg sensoready pen.
Cosentyx Srf Form Fill Online, Printable, Fillable, Blank pdfFiller
Download the npaf application form english (pdf 0.1 mb) | spanish (pdf 0.1 mb) Web cosentyx 150 mg sensoready pen (1x150 mg/ml) prefilled syringe (1x150 mg/ml) adult: Talk to your doctor about submitting an appeal to the. Cosentyx ® 1 844 267 3689. In the meantime, here’s what you can do:
Cosentyx 300mg Dose Injections 13 & 14 YouTube
Web request form for cosentyx, and be experiencing a delay in obtaining coverage. Cosentyx is indicated for the treatment. Sign up now for access to a full range of services and support that are. Beovu ® 1 888 612 3688. Web cosentyx ® connect enrollment tearsheet a checklist to help patients understand the process of receiving their cosentyx prescription and.
Cosentyx 6 Month Update Psoriasis YouTube
Your patients don't have to wait for their first dose of cosentyx to start taking advantage of all the tools and services available: Web novartis patient support contacts. Web cosentyx 150 mg sensoready pen (1x150 mg/ml) prefilled syringe (1x150 mg/ml) adult: Ad learn how cosentyx treats multiple conditions & see if it is right for you. If a serious infection.
Incredible Cosentyx Start Form 2022 · News
Web cosentyx ® connect enrollment tearsheet a checklist to help patients understand the process of receiving their cosentyx prescription and the resources available to them,. Your patients don't have to wait for their first dose of cosentyx to start taking advantage of all the tools and services available: Learn more about cosentyx® & how to get your patients started today..
DepEd Automated Modified Learner Enrollment and Survey Form (MLESF) for
Send out signed cosentyx enrollment form 2022 pdf or print it. Web novartis patient support contacts. Mayzent ® 1 877 629 9368. Web when considering the use of cosentyx in patients with a chronic infection or a history of recurrent infection. If a serious infection develops, discontinue cosentyx until the.
Cosentyx ® 1 844 267 3689.
Web xpose® program enrollment and consent form: Cosentyx is indicated for the treatment. Web enroll now just considering cosentyx? Customize and esign cosentyx start form.
Learn More About Cosentyx® & How To Get Your Patients Started Today.
Program provides initial 5 weekly doses (if prescribed) and monthly doses for free to patients for. Sign up for cosentyx connect at. Web when considering the use of cosentyx in patients with a chronic infection or a history of recurrent infection. Web cosentyx 150 mg sensoready pen (1x150 mg/ml) prefilled syringe (1x150 mg/ml) adult:
Talk To Your Doctor About Submitting An Appeal To The.
Ad learn how cosentyx treats multiple conditions & see if it is right for you. Learn more about cosentyx® & how to get your patients started today. Your patients don't have to wait for their first dose of cosentyx to start taking advantage of all the tools and services available: If a serious infection develops, discontinue cosentyx until the.
Sign Up Now For Access To A Full Range Of Services And Support That Are.
Omnitrope ® 1 877 456 6794. Ad view prescribing, safety & efficacy information for hcps on cosentyx®. Send out signed cosentyx enrollment form 2022 pdf or print it. Web up to $40 cash back cosentyx srf (successor registration form) is a form that is required to be submitted for patients who have switched from the innovator product to a biosimilar.