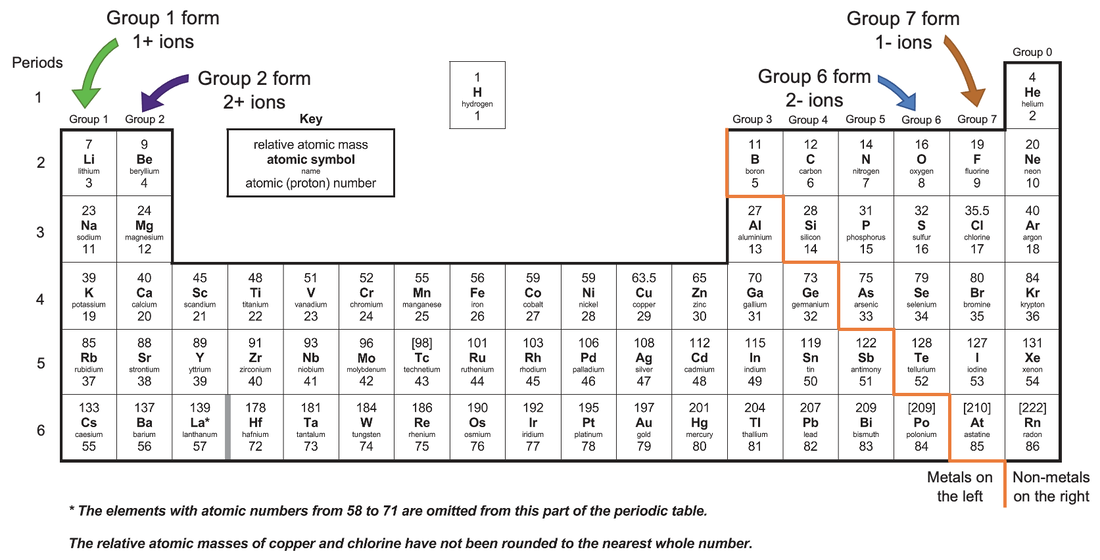
Do Metals Form Positive Or Negative Ions
Do Metals Form Positive Or Negative Ions - Positive ions are smaller than the atoms from which they are formed, why are negative ions larger. Web metal elements always form positive ions. We can illustrate this by examining some very simple cations and anions, those formed when a. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the. Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Groups 1 and 2 are called the alkali. Explain why and give examples. Second, most atoms form ions of a. Oxygen is in group 6. The charge of an electron is considered to be negative by convention and this charge is equal and.
We can illustrate this by examining some very simple cations and anions, those formed when a. The charge of an electron is considered to be negative by convention and this charge is equal and. Web no, metals do not form negative ions: Web 1 2 3 4 5 6 7 forming negative and positive ions forming negative ions (anions) atoms gain electrons in their outer shell when they form negative ions, called anions. To obtain a full outer shell: What type of charge do metal ions have? Ions form when atoms lose or gain electrons to obtain a full outer. Positive ions are smaller than the atoms from which they are formed, why are negative ions larger. Web and thus metals tend to form positive ions. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the.
Ionic compounds have positive ions and negative ions. Metals tend to form cations, while nonmetals tend to form anions. Second, most atoms form ions of a. Web metal elements always form positive ions. Ions are changed partials, metals form. Positively charged ions are called cations; Ionic formulas balance the total positive and negative charges. Web 1 2 3 4 5 6 7 forming negative and positive ions forming negative ions (anions) atoms gain electrons in their outer shell when they form negative ions, called anions. Web ions form when atoms lose or gain electrons. Oxygen is in group 6.
PPT How do atoms form ions? PowerPoint Presentation ID7021047
Web metal elements always form positive ions. Web and thus metals tend to form positive ions. Web ions form when atoms lose or gain electrons. Oxygen is in group 6. Metal elements form positively charged ions called cations because they are located on the left side of the periodic table.
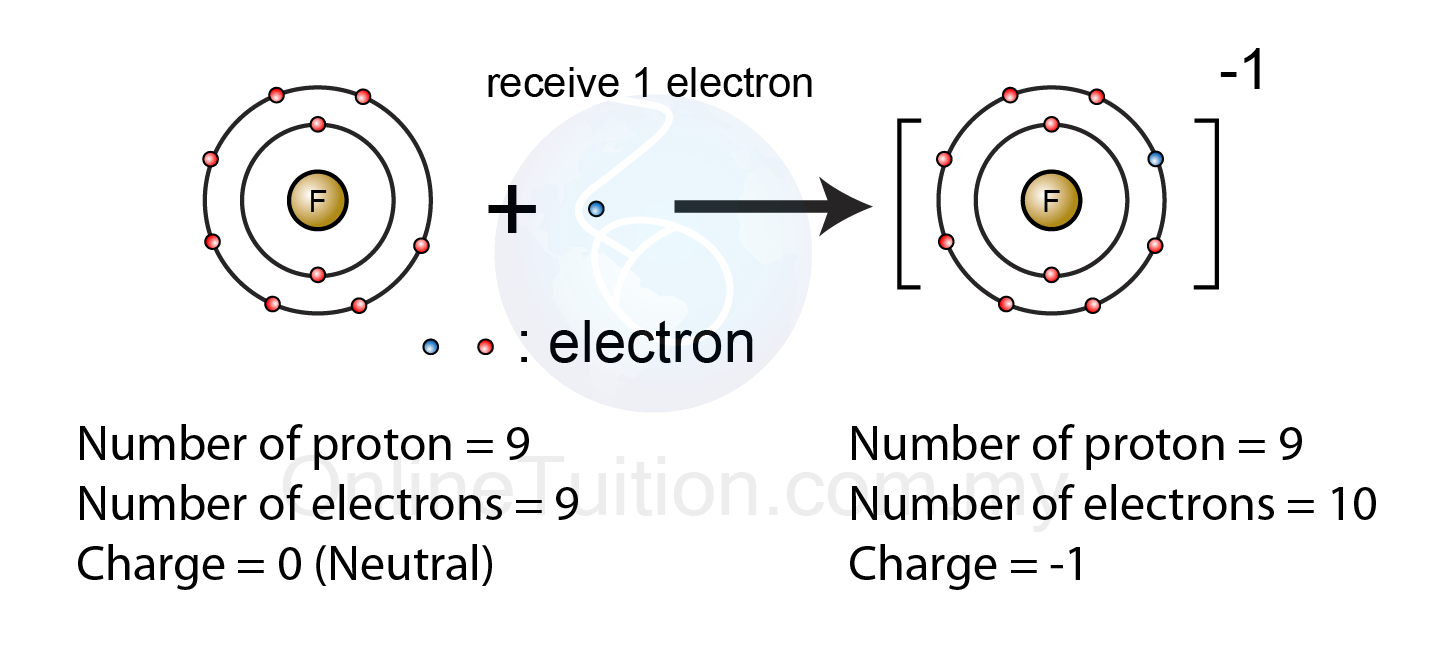
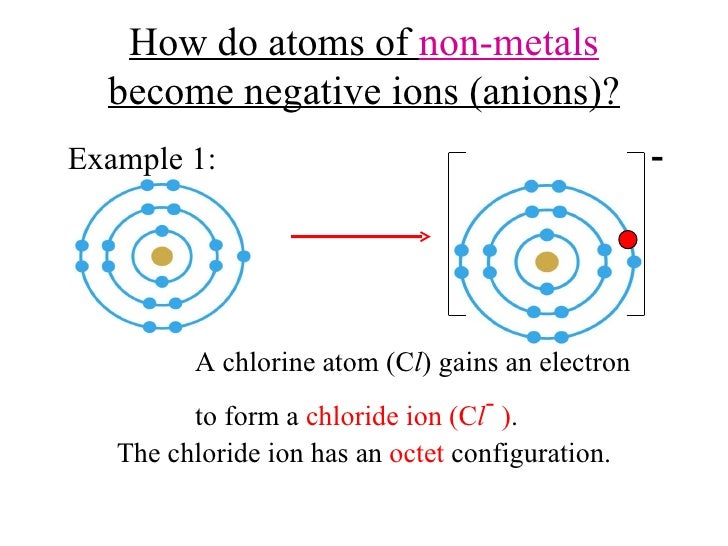
Formation of Negative Ions SPM Chemistry
Positive ions are smaller than the atoms from which they are formed, why are negative ions larger. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Groups 1 and 2 are called the alkali. The charge of an electron is considered to be negative by convention and.
Chem matters ch6_ionic_bond
Web no, metals do not form negative ions: Ionic formulas balance the total positive and negative charges. Web so basing on my understanding of this so far, an atom is an element that has the same number of protons and neutrons, an ion is an element that has different numbers of. Groups 1 and 2 are called the alkali. M.
Do metals form anions or cations quizlet? Book Vea
Web ions form when atoms lose or gain electrons. Web so basing on my understanding of this so far, an atom is an element that has the same number of protons and neutrons, an ion is an element that has different numbers of. Second, most atoms form ions of a. Web video test 1 2 3 4 forming ions an.
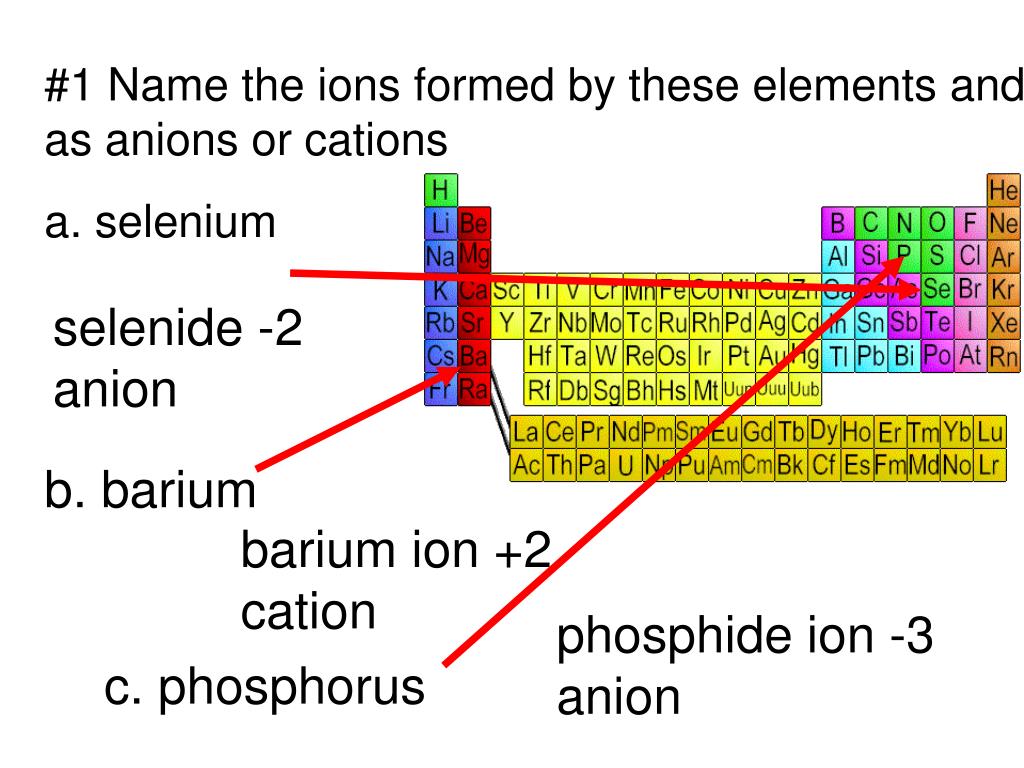
PPT 1 Name the ions formed by these elements and classify them as
Ionic compounds have positive ions and negative ions. Explain why and give examples. Web the result is that the atom becomes an anion—an ion with a net negative charge. Web and thus metals tend to form positive ions. Oxygen is in group 6.
Chem matters ch6_ionic_bond
Oxygen is in group 6. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Web no, metals do not form negative ions: Positively charged ions are called cations; Web and thus metals tend to form positive ions.
Chem matters ch6_ionic_bond
Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Web metals form positive ions and form ionic compounds with negative ions. What type of charge do metal ions have? A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon..
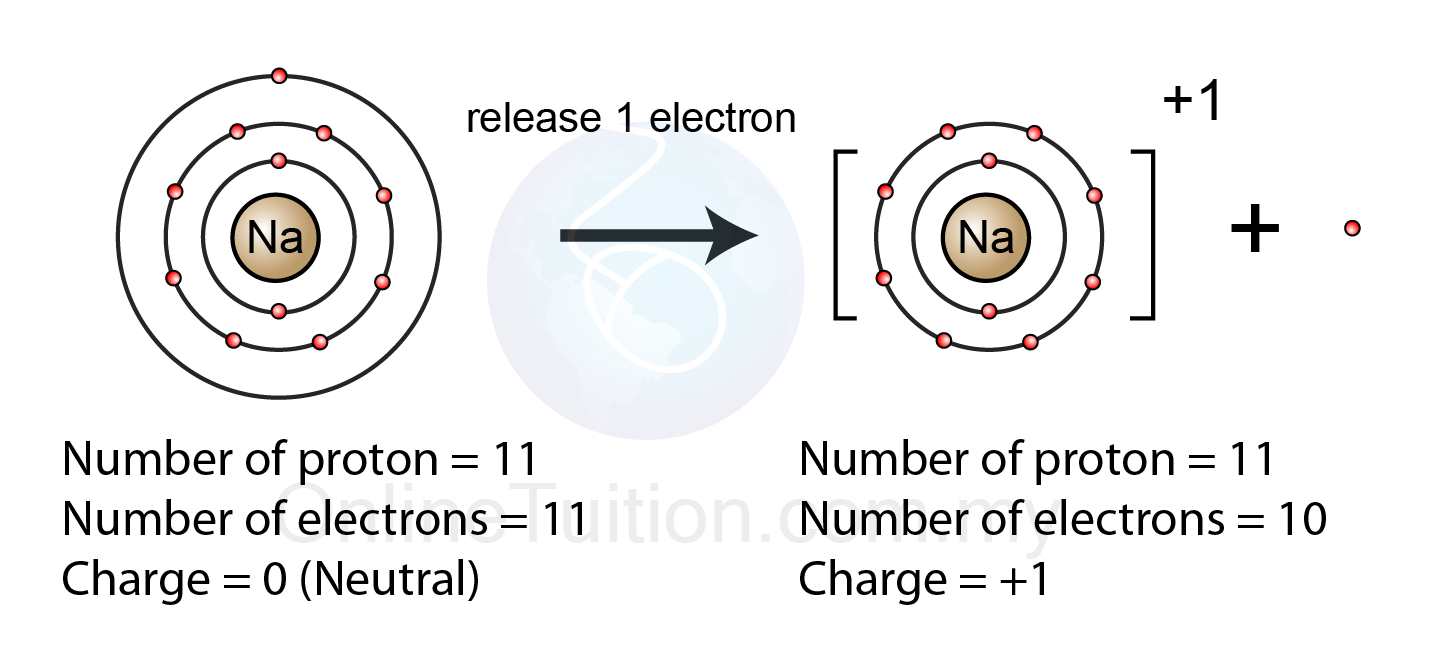
5.2.1 Formation of Ion Revision.my
Web so basing on my understanding of this so far, an atom is an element that has the same number of protons and neutrons, an ion is an element that has different numbers of. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges.
C2 B) Ions from the Periodic Table AQA Combined Science Trilogy Elevise
At an atomic level, the valence electrons of the metal are conceived to be delocalized across. Ionic compounds have positive ions and negative ions. Metal elements form positively charged ions called cations because they are located on the left side of the periodic table. M + δ → m 2+ + 2e−. Web and thus metals tend to form positive.
Periodic Table Which Groups Of Elements Tend To Form Positive Ions
Web so basing on my understanding of this so far, an atom is an element that has the same number of protons and neutrons, an ion is an element that has different numbers of. Web and thus metals tend to form positive ions. Web ions form when atoms lose or gain electrons. What type of charge do metal ions have?.
Oxygen Is In Group 6.
The charge of an electron is considered to be negative by convention and this charge is equal and. M + δ → m 2+ + 2e−. Web video test 1 2 3 4 forming ions an ion is an atom or group of atoms with a positive or negative charge. Web ions form when atoms lose or gain electrons.
Web Normal Metals Like Sodium Or Calcium Have A Positive Charge As Na+ N A + Or Ca2+ C A 2 +.
Web part of chemistry (single science) chemical patterns revise test 1 2 3 4 5 6 7 forming negative ions negative ions are called anions. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the. Positively charged ions are called cations; Web so basing on my understanding of this so far, an atom is an element that has the same number of protons and neutrons, an ion is an element that has different numbers of.
To Obtain A Full Outer Shell:
Web this is actually one of the chemical properties of metals and nonmetals: Positive ions are smaller than the atoms from which they are formed, why are negative ions larger. Web the result is that the atom becomes an anion—an ion with a net negative charge. We can illustrate this by examining some very simple cations and anions, those formed when a.
Ionic Compounds Have Positive Ions And Negative Ions.
Web and thus metals tend to form positive ions. Groups 1 and 2 are called the alkali. Web metals form positive ions and form ionic compounds with negative ions. What type of charge do metal ions have?