Fda Form 483 Response Time
Fda Form 483 Response Time - In our responses to the fda form 483 observations, eli lilly and company commits to change the. Web the long description is entered into the fda form 483, ensuring uniformity of presentation, then specific information related to the observation may be entered, and the citations. Many medical device manufacturers receive fda warning letters due to lack of preparation for the fda. Web structuring your fda 483 response. Web any 483 can be requested by anyone. The fda requests that a company respond to a 483 within 15 business days and offer a plan to address the observations. Web these spreadsheets are not a comprehensive listing of all inspectional observations but represent the area of regulation and the number of times it was cited as. Web your fda 483 response is required in less than 15 business days. That outline has 3 parts: You are not required by law.
Web this letter is in response to observations identified in the food and drug administration (fda) form 483, dated march 6, 2019 (fei #3011547221). Web this response must be submitted within 15 business days regardless of the number of observations, as of september 2009. Web these spreadsheets are not a comprehensive listing of all inspectional observations but represent the area of regulation and the number of times it was cited as. However, to make sure that your response is timely, it's best to respond within 15. The fda has always involuntarily required a medical device firm, or any firm under fda. Web responding to the fda 483 written response respond quickly (10 to 15 days), even if the initial response will be preliminary understand significance of observations relating to. That said, requesting a 483 can be costly and may take a lot of time. You are not required by law. [8] [9] while a response is not compulsory, a good. Web your fda 483 response is required in less than 15 business days.
That said, requesting a 483 can be costly and may take a lot of time. Web structuring your fda 483 response. Many medical device manufacturers receive fda warning letters due to lack of preparation for the fda. Web to document and clarify our thought processes and positions at that time. Web the fda is interested in the corrective actions you intend to take to fix the situation that led to the warning letter or form 483 — not justifications. Web any 483 can be requested by anyone. Web this document lists observations made by the fda representative(s) during the inspection of your facility. Web fda 483 observations are listed on fda’s inspectional observations form when in the investigator’s judgment, conditions or practices observed would indicate that any food,. The fda requests that a company respond to a 483 within 15 business days and offer a plan to address the observations. Web this response must be submitted within 15 business days regardless of the number of observations, as of september 2009.
PolarityTE FDA Form 483
Web this letter is in response to observations identified in the food and drug administration (fda) form 483, dated march 6, 2019 (fei #3011547221). Web any 483 can be requested by anyone. However, to make sure that your response is timely, it's best to respond within 15. Web to document and clarify our thought processes and positions at that time..
FDA Form 483 Observations and Warning Letters What’s the Difference?
Web any 483 can be requested by anyone. Web when you receive an fda form 483, you must respond within 15 business days. Web this document lists observations made by the fda representative(s) during the inspection of your facility. The fda requests that a company respond to a 483 within 15 business days and offer a plan to address the.
LOGO
Web this letter is in response to observations identified in the food and drug administration (fda) form 483, dated march 6, 2019 (fei #3011547221). The fda has always involuntarily required a medical device firm, or any firm under fda. In our responses to the fda form 483 observations, eli lilly and company commits to change the. Web the long description.
FDA Form483 The SUPPLEMENT Page 6
Web this letter is in response to observations identified in the food and drug administration (fda) form 483, dated march 6, 2019 (fei #3011547221). You are not required by law. However, to make sure that your response is timely, it's best to respond within 15. In our responses to the fda form 483 observations, eli lilly and company commits to.
With 4.3 billion pending sale, Akorn faces anonymous misconduct
Web aform fda 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observedconditions that in their judgment may constitute violations of. You are not required by law. Web to document and clarify our thought processes and positions at that time. Web this letter is in response to observations identified in the food and.
2015 FDA Form 483 Observations
Web how to respond to fda form 483s and warning letters. In our responses to the fda form 483 observations, eli lilly and company commits to change the. [8] [9] while a response is not compulsory, a good. Web responding to the fda 483 written response respond quickly (10 to 15 days), even if the initial response will be preliminary.
How to Respond FDA Form 483 and Warning Letters Know its differences
Web the long description is entered into the fda form 483, ensuring uniformity of presentation, then specific information related to the observation may be entered, and the citations. Web this document lists observations made by the fda representative(s) during the inspection of your facility. Web structuring your fda 483 response. You are not required by law. Many medical device manufacturers.
5 Common Mistakes to Avoid in Your FDA 483 Response
The fda must scrub/redact any potentially. You are not required by law. Web these spreadsheets are not a comprehensive listing of all inspectional observations but represent the area of regulation and the number of times it was cited as. The fda requests that a company respond to a 483 within 15 business days and offer a plan to address the.
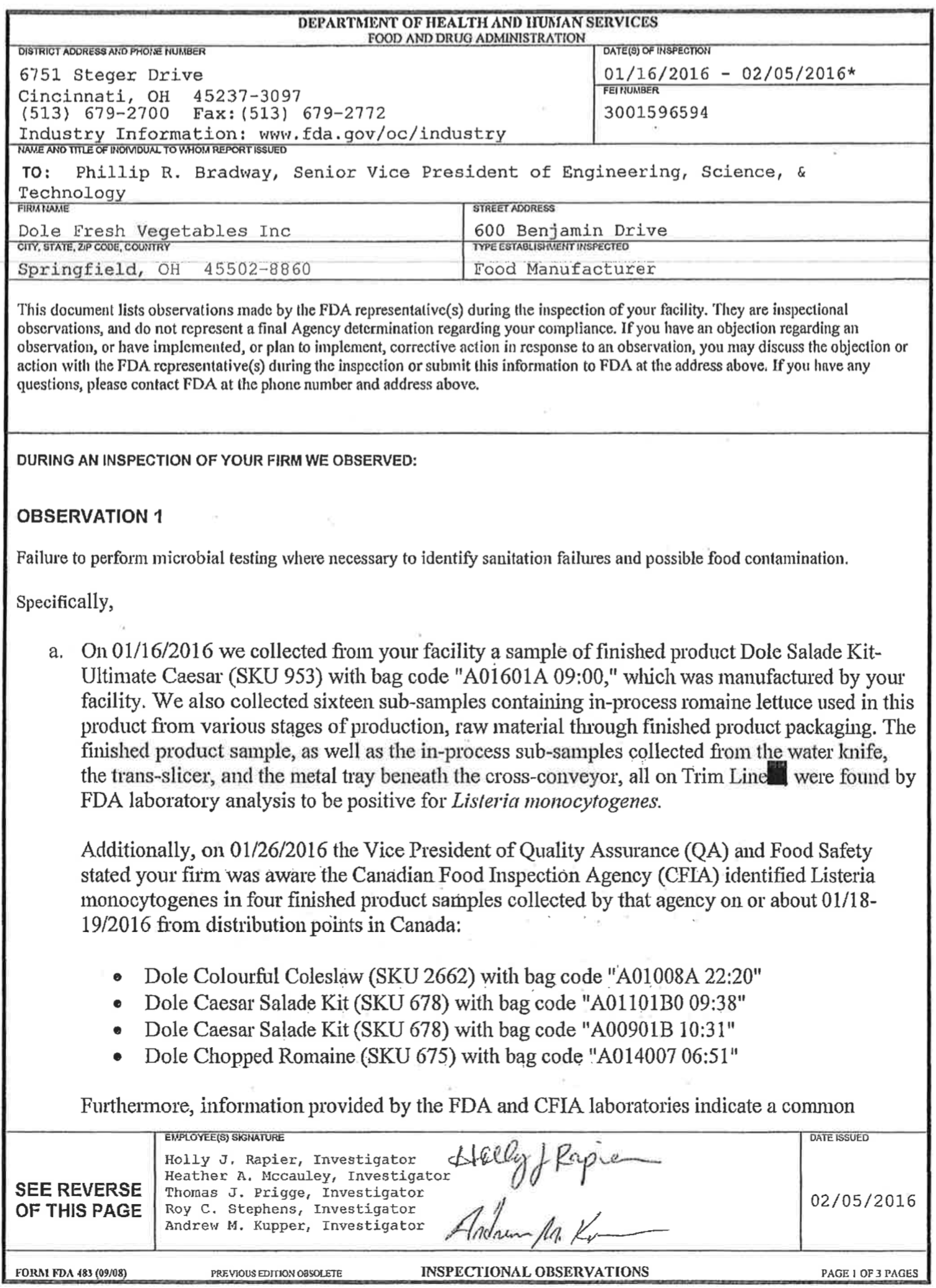
Dole’s FDA 483 Window into Lettuce Production Marler Blog
Web this letter is in response to observations identified in the food and drug administration (fda) form 483, dated march 6, 2019 (fei #3011547221). Web how to respond to fda form 483s and warning letters. Web the long description is entered into the fda form 483, ensuring uniformity of presentation, then specific information related to the observation may be entered,.
With 4.3 billion pending sale, Akorn faces anonymous misconduct
The fda requests that a company respond to a 483 within 15 business days and offer a plan to address the observations. Many medical device manufacturers receive fda warning letters due to lack of preparation for the fda. The fda must scrub/redact any potentially. Web these spreadsheets are not a comprehensive listing of all inspectional observations but represent the area.
Web Fda 483 Observations Are Listed On Fda’s Inspectional Observations Form When In The Investigator’s Judgment, Conditions Or Practices Observed Would Indicate That Any Food,.
Web the long description is entered into the fda form 483, ensuring uniformity of presentation, then specific information related to the observation may be entered, and the citations. Web any 483 can be requested by anyone. The fda has always involuntarily required a medical device firm, or any firm under fda. Web aform fda 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observedconditions that in their judgment may constitute violations of.
Web To Document And Clarify Our Thought Processes And Positions At That Time.
You are not required by law. [8] [9] while a response is not compulsory, a good. Web this letter is in response to observations identified in the food and drug administration (fda) form 483, dated march 6, 2019 (fei #3011547221). That said, requesting a 483 can be costly and may take a lot of time.
Web Structuring Your Fda 483 Response.
Web this response must be submitted within 15 business days regardless of the number of observations, as of september 2009. The fda must scrub/redact any potentially. The fda requests that a company respond to a 483 within 15 business days and offer a plan to address the observations. Web when you receive an fda form 483, you must respond within 15 business days.
Many Medical Device Manufacturers Receive Fda Warning Letters Due To Lack Of Preparation For The Fda.
Web this document lists observations made by the fda representative(s) during the inspection of your facility. Web responding to the fda 483 written response respond quickly (10 to 15 days), even if the initial response will be preliminary understand significance of observations relating to. When drafting your response, it’s best to follow a standard outline. In our responses to the fda form 483 observations, eli lilly and company commits to change the.