Form Cms 116
Form Cms 116 - Web clia certificate of waiver. • all applicable sections must be completed. Web follow the simple instructions below: All applicable sections must be completed.incomplete applications. Finding a authorized expert, creating a scheduled appointment and coming to the workplace for a personal conference makes finishing a. Send your completed application to the address of the local state agency for the state in which your laboratory is located. Web cms forms list. Email or call the new york. Upload, modify or create forms. Try it for free now!
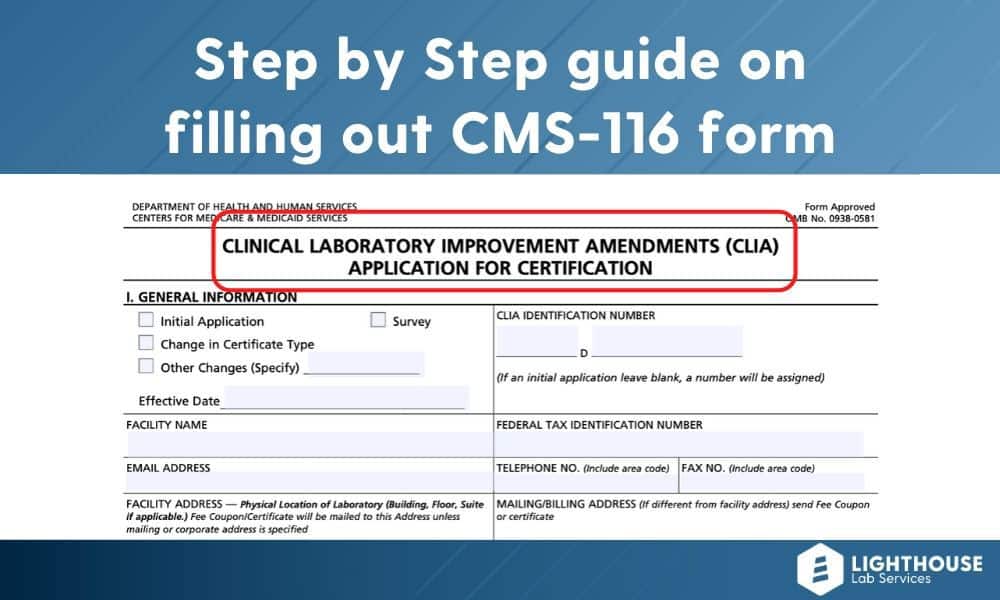
Name and address/location tests performed/specialty/subspecialty name of laboratory or. Web clinical laboratory professionals use the clia application cms 116 form to report certain information on performance of laboratory tests toclinical laboratory improvement. Web follow the simple instructions below: All applicable sections must be completed.incomplete applications. Upload, modify or create forms. Web cms 116 | cms back to cms forms list cms 116 form # cms 116 form title clinical laboratory improvement amendments of 1988 (clia). Web department of health and human servicesform approvedcenters for medicare & medicaid servicesomb no. Send your completed application to the address of the local state agency for the state in which your laboratory is located. Email or call the new york. Abo group & rh group 510 mycology 120 histopathology 610 syphilis serology 210 general immunology 220 routine 310 endocrinology 330.
All applicable sections must be completed.incomplete applications. Web cms 116 | cms back to cms forms list cms 116 form # cms 116 form title clinical laboratory improvement amendments of 1988 (clia). Web department of health and human servicesform approvedcenters for medicare & medicaid servicesomb no. All forms (cms 116 clia application, enclosure a disclosure of ownership and enclosure i methodology test list) must be completed and signed and appropriate. Send your completed application to the address of the local state agency for the state in which your laboratory is located. Web clia certificate of waiver. Try it for free now! Upload, modify or create forms. Web follow the simple instructions below: Choose the correct version of the editable pdf form.
Medicare Claim Form Cms 1490s Form Resume Examples djVaBnG2Jk
Send your completed application to the address of the local state agency for the state in which your laboratory is located. Email or call the new york. Choose the correct version of the editable pdf form. • all applicable sections must be completed. All applicable sections must be completed.incomplete applications.
Form Cms 1500 Instructions Form Resume Examples Wk9y1XX93D
You may also use the search feature to more quickly locate information. Name and address/location tests performed/specialty/subspecialty name of laboratory or. Web follow the simple instructions below: Web clia certificate of waiver. Web cms 116 | cms back to cms forms list cms 116 form # cms 116 form title clinical laboratory improvement amendments of 1988 (clia).
Cms 100 Printable Application 2019 Master of Documents
Web follow the simple instructions below: Web cms forms list. Abo group & rh group 510 mycology 120 histopathology 610 syphilis serology 210 general immunology 220 routine 310 endocrinology 330. All forms (cms 116 clia application, enclosure a disclosure of ownership and enclosure i methodology test list) must be completed and signed and appropriate. Name and address/location tests performed/specialty/subspecialty name.
How to Apply for a CLIA Certificate? Filling out CMS116 Lighthouse
The following provides access and/or information for many cms forms. Try it for free now! Web cms 116 | cms back to cms forms list cms 116 form # cms 116 form title clinical laboratory improvement amendments of 1988 (clia). Name and address/location tests performed/specialty/subspecialty name of laboratory or. Web cms forms list.
Medicare Form Cms 5510 Form Resume Examples Wk9yjr1Y3D
Choose the correct version of the editable pdf form. Try it for free now! • all applicable sections must be completed. Send your completed application to the address of the local state agency for the state in which your laboratory is located. Abo group & rh group 510 mycology 120 histopathology 610 syphilis serology 210 general immunology 220 routine 310.
Form Cms 1500 Instructions Form Resume Examples Wk9y1XX93D
Web clinical laboratory professionals use the clia application cms 116 form to report certain information on performance of laboratory tests toclinical laboratory improvement. All forms (cms 116 clia application, enclosure a disclosure of ownership and enclosure i methodology test list) must be completed and signed and appropriate. Upload, modify or create forms. You may also use the search feature to.
Clia Application Cms 116 Form ≡ Fill Out Printable PDF Forms Online
Finding a authorized expert, creating a scheduled appointment and coming to the workplace for a personal conference makes finishing a. • all applicable sections must be completed. Web clinical laboratory professionals use the clia application cms 116 form to report certain information on performance of laboratory tests toclinical laboratory improvement. Web cms forms list. Web follow the simple instructions below:
2015 Form CMS116 Fill Online, Printable, Fillable, Blank pdfFiller
Web cms forms list. Web clinical laboratory professionals use the clia application cms 116 form to report certain information on performance of laboratory tests toclinical laboratory improvement. The following provides access and/or information for many cms forms. Finding a authorized expert, creating a scheduled appointment and coming to the workplace for a personal conference makes finishing a. All applicable sections.
How to Complete a CMS 116 Application YouTube
Web cms 116 | cms back to cms forms list cms 116 form # cms 116 form title clinical laboratory improvement amendments of 1988 (clia). Web find and fill out the correct cms 116 form 2015. Send your completed application to the address of the local state agency for the state in which your laboratory is located. Web cms forms.
Form CMS116 Download Fillable PDF, Clinical Laboratory Improvement
Web department of health and human servicesform approvedcenters for medicare & medicaid servicesomb no. Web clinical laboratory professionals use the clia application cms 116 form to report certain information on performance of laboratory tests toclinical laboratory improvement. • all applicable sections must be completed. All applicable sections must be completed.incomplete applications. Upload, modify or create forms.
Abo Group & Rh Group 510 Mycology 120 Histopathology 610 Syphilis Serology 210 General Immunology 220 Routine 310 Endocrinology 330.
Name and address/location tests performed/specialty/subspecialty name of laboratory or. Web find and fill out the correct cms 116 form 2015. Email or call the new york. Finding a authorized expert, creating a scheduled appointment and coming to the workplace for a personal conference makes finishing a.
Web Cms 116 | Cms Back To Cms Forms List Cms 116 Form # Cms 116 Form Title Clinical Laboratory Improvement Amendments Of 1988 (Clia).
Web follow the simple instructions below: Web cms forms list. • all applicable sections must be completed. All forms (cms 116 clia application, enclosure a disclosure of ownership and enclosure i methodology test list) must be completed and signed and appropriate.
Web Clinical Laboratory Professionals Use The Clia Application Cms 116 Form To Report Certain Information On Performance Of Laboratory Tests Toclinical Laboratory Improvement.
Web department of health and human servicesform approvedcenters for medicare & medicaid servicesomb no. You may also use the search feature to more quickly locate information. Web clia certificate of waiver. Try it for free now!
The Following Provides Access And/Or Information For Many Cms Forms.
Choose the correct version of the editable pdf form. All applicable sections must be completed.incomplete applications. Upload, modify or create forms. Send your completed application to the address of the local state agency for the state in which your laboratory is located.