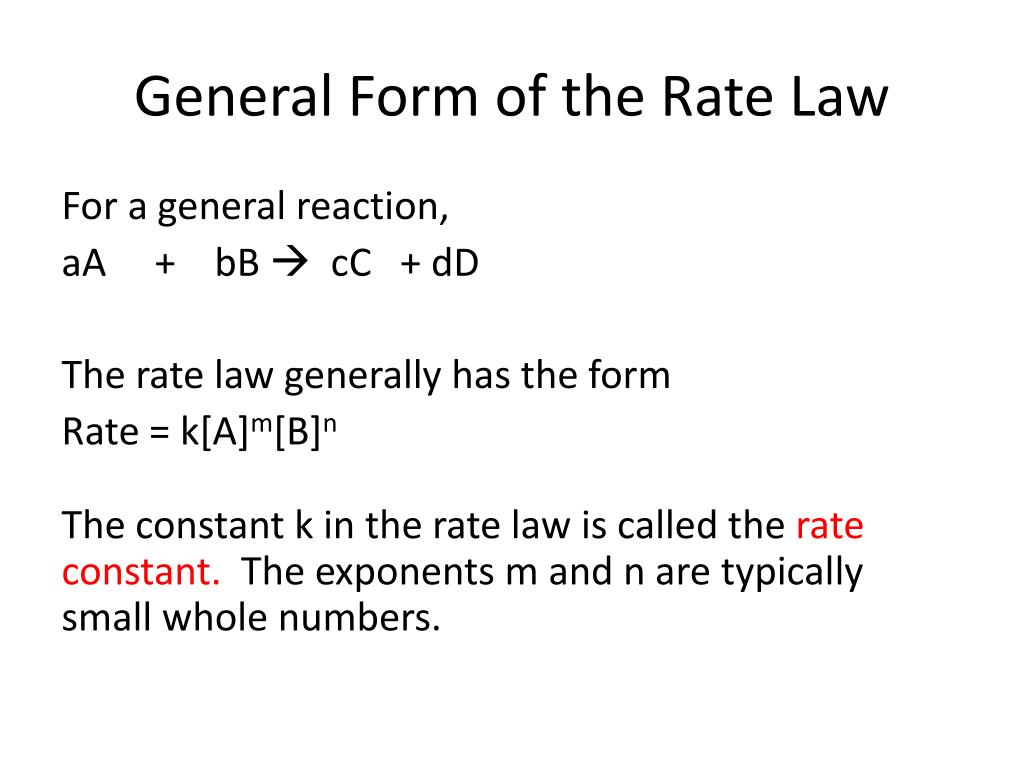
General Form Of A Rate Law
General Form Of A Rate Law - Web according to the law of mass action, the rate of a chemical reaction at a constant temperature depends only on the concentrations of the substances that. The rate law (also known as the rate equation) for a chemical reaction is an expression that provides a relationship between the rate of the reaction. The equation which gives exact mathematical relation between rate of… q:. 100% (12 ratings) general form of rate law, rate = k [a]^x [b]^y where, k = ra. Web a differential rate law expresses the rate of a reaction in terms of changes in the concentration of reactants over a specific interval of time. Web rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical reaction and the concentration of its reactants. Web still, revenue from reels is growing, reaching an annual sales rate of $10 billion, zuckerberg said, up from $3 billion in the third quarter of 2022. The differential rate law is. Web this article provides information and answers on powers of attorney in texas. Use rate and concentration data to identify reaction orders and derive rate laws.
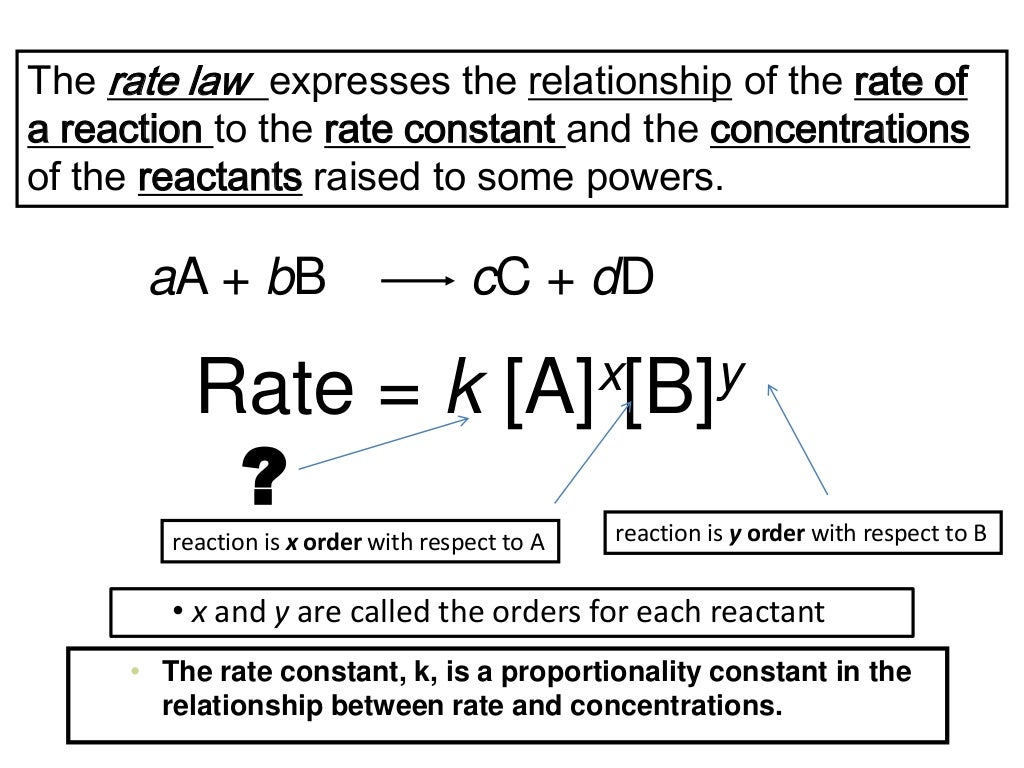
The equation which gives exact mathematical relation between rate of… q:. The most general description of a chemical reaction network considers a number of distinct chemical species reacting via reactions. Web the rate law of a chemical reaction is an expression that provides a relationship between the rate of the reaction and the concentrations of the reactants. Web these are called integrated rate laws. Which statement is true for the general rate law, rate = k[a][b] %3d a: Rate laws can be expressed in either. An equation relating the rate of a chemical reaction to the concentrations or partial pressures of the reactants. Rate=k [a]m [b]n [c]p… rate = k [ a ] m [ b ] n [ c ] p. Start with the general rate law. Use rate and concentration data to identify reaction orders and derive rate laws.
Is reaction rate, expressed in concentration/unit of time (usually = molarity/second) is the specific rate constant and are. Use rate and concentration data to identify reaction orders and derive rate laws. Web in the standard form, the rate law equation is written as: Web according to the law of mass action, the rate of a chemical reaction at a constant temperature depends only on the concentrations of the substances that. 100% (12 ratings) general form of rate law, rate = k [a]^x [b]^y where, k = ra. Web explain the form and function of a rate law. Integration of the differential rate law yields the concentration as a function of time. You'll get a detailed solution from a subject matter expert that helps you. Web write the genral form of the rate law for each of the following reactions this problem has been solved! Rate law (or) rate equation:
8.1 rate law
The rate law for a chemical reaction is an equation that. Web what is the general form of a rate law? Web in the standard form, the rate law equation is written as: Web this article provides information and answers on powers of attorney in texas. Rate law (or) rate equation:
PPT The Rate Law PowerPoint Presentation, free download ID5857354
Here, learn about texas' different types of powers of attorney, including general, limited,. Rate laws can be expressed in either. Well, you are given the mechanism for the overall reaction, so you cannot say that the rate law is r(t) = k[n o2]2[f 2]. Rate=k [a]m [b]n [c]p… rate = k [ a ] m [ b ] n [.
The type of rate law for a reaction, either the d…
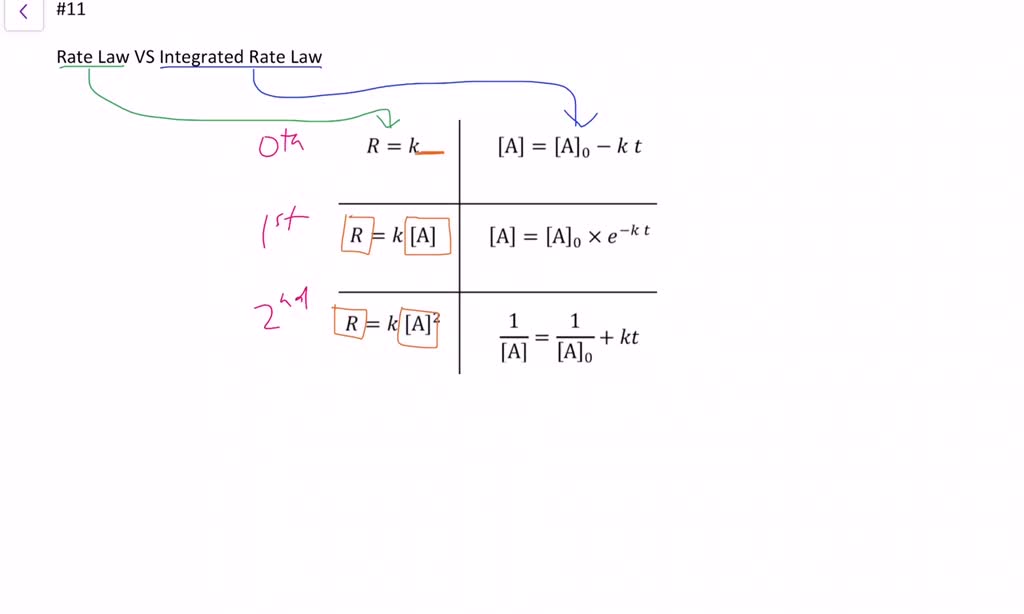
The differential rate law is. The rate law (also known as the rate equation) for a chemical reaction is an expression that provides a relationship between the rate of the reaction. Is reaction rate, expressed in concentration/unit of time (usually = molarity/second) is the specific rate constant and are. The most general description of a chemical reaction network considers a.
8.1 rate law
Which statement is true for the general rate law, rate = k[a][b] %3d a: Web integrated form of the zeroth order rate law. Rate laws can be expressed in either. Web rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical reaction and the concentration of its reactants. Web july 19, 2023.
Integrated Rate Law Problems 1 YouTube
Use rate and concentration data to identify reaction orders and derive rate laws. Rate law (or) rate equation: Integration of the differential rate law yields the concentration as a function of time. Rate laws can be expressed in either. Web integrated form of the zeroth order rate law.
Solving a Rate Law Using the Initial Rates Method YouTube
Start with the general rate law. Web july 19, 2023. Here, learn about texas' different types of powers of attorney, including general, limited,. We can use an integrated rate law to determine the amount of reactant or product present after a period of time or to estimate. Web these are called integrated rate laws.
13.2 The Rate Law YouTube
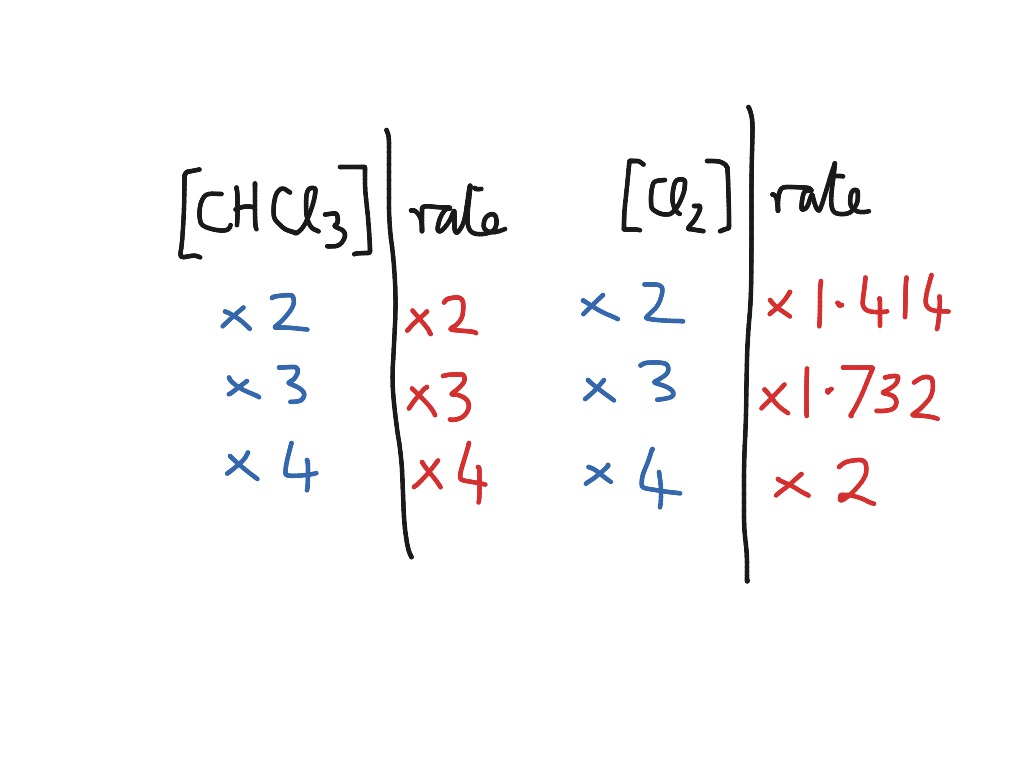
Rate = k [a]^x [b]^y it is found that for the reaction a+b —> c that doubling the concentration of either a or b quadruples the rate of the. Which statement is true for the general rate law, rate = k[a][b] %3d a: Web a differential rate law expresses the rate of a reaction in terms of changes in the.
A rate law example Science ShowMe
Web this article provides information and answers on powers of attorney in texas. Which statement is true for the general rate law, rate = k[a][b] %3d a: We can use an integrated rate law to determine the amount of reactant or product present after a period of time or to estimate. Web explain the form and function of a rate.
Rate Law YouTube
An equation relating the rate of a chemical reaction to the concentrations or partial pressures of the reactants. Use rate laws to calculate reaction rates. Start with the general rate law. Integration of the differential rate law yields the concentration as a function of time. Web explain the form and function of a rate law.
Introduction to the Rate Law YouTube
Which statement is true for the general rate law, rate = k[a][b] %3d a: 100% (12 ratings) general form of rate law, rate = k [a]^x [b]^y where, k = ra. Well, you are given the mechanism for the overall reaction, so you cannot say that the rate law is r(t) = k[n o2]2[f 2]. Web still, revenue from reels.
Web In The Standard Form, The Rate Law Equation Is Written As:
Web write the genral form of the rate law for each of the following reactions this problem has been solved! An equation relating the rate of a chemical reaction to the concentrations or partial pressures of the reactants. The rate law (also known as the rate equation) for a chemical reaction is an expression that provides a relationship between the rate of the reaction. Web what is the rate law?
Which Statement Is True For The General Rate Law, Rate = K[A][B] %3D A:
Web this article provides information and answers on powers of attorney in texas. Web july 19, 2023. Integration of the differential rate law yields the concentration as a function of time. Web explain the form and function of a rate law.
The Differential Rate Law Is.
Web according to the law of mass action, the rate of a chemical reaction at a constant temperature depends only on the concentrations of the substances that. Is reaction rate, expressed in concentration/unit of time (usually = molarity/second) is the specific rate constant and are. The rate law for a chemical reaction is an equation that. You'll get a detailed solution from a subject matter expert that helps you.
Web In General, A Rate Law (Or Differential Rate Law, As It Is Sometimes Called) Takes This Form:
The most general description of a chemical reaction network considers a number of distinct chemical species reacting via reactions. Rate=k [a]m [b]n [c]p… rate = k [ a ] m [ b ] n [ c ] p. Start with the general rate law. Web rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical reaction and the concentration of its reactants.