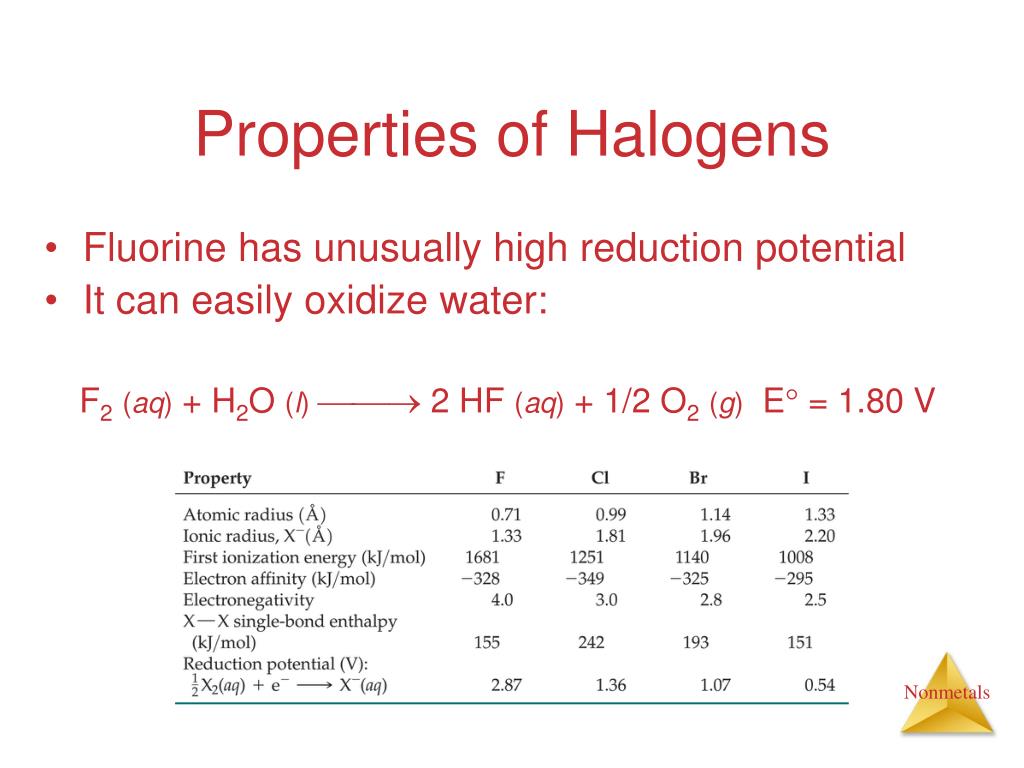
Halogens Tend To Form Anions Because
Halogens Tend To Form Anions Because - C) anions are usually larger than their corresponding atom. D) halogen atomic radii tend to be larger than their corresponding ionic radii. B) gaining electrons will fill their octet faster than losing them. Why does halogen tend to form an anion? Web halogens (flourine, chlorine, bromine, iodine, astatine) and oxygen tend to form anions when bonding with other elements. Well, your question might actually require a direct answer but let me try expounding it in a better understandable way. Web generally speaking the elements in the top right side of the periodic table (the nonmetals and halogens) tend to form anions. Web 68) a) atoms are usually larger than their corresponding cation b) the halogens tend to form 1+ ions. However, you aren't likely to see a naked xx+ x x +. To begin with, halogens are those elements found in group 17 ( fomerly group 7) of the periodic table and they include;
Web halogens tend to form anions because a) losing electrons will fill their octet faster than gaining them. The middle halogens—chlorine, bromine, and. Choose the false statement ( read carefully ) a. As expected, these elements have certain properties in common. Web halogenation is a term that refers to a chemical process that involves the addition of one or more halogens to a substance or a compound. Web the halogens tend to form 1+ ions because they only need one electron to fill their valence shell. C) anions are usually larger than their corresponding atom. However, you aren't likely to see a naked xx+ x x +. Most halogens are typically produced from minerals or salts. Web generally speaking the elements in the top right side of the periodic table (the nonmetals and halogens) tend to form anions.
Web all of the halogens form acids when bonded to hydrogen. 1) they give up one electron to achieve the octet rule. Web halogens (flourine, chlorine, bromine, iodine, astatine) and oxygen tend to form anions when bonding with other elements. Well, your question might actually require a direct answer but let me try expounding it in a better understandable way. D) halogen atomic radii tend to be larger than their corresponding ionic radii. Web halogens tend to form anions because a) losing electrons will fill their octet faster than gaining them. Halogens tend to form anions because a) losing electrons will fill their octet faster than gaining them. The stoichiometry and route of. Most halogens are typically produced from minerals or salts. 2) have 7 valence electrons and need one more.
anion Common anions, their names, formulas and the elements they are
Web the halogens tend to form 1+ ions because they only need one electron to fill their valence shell. Web halogens tend to form anions because a) losing electrons will fill their octet faster than gaining them. Web se cr ar ar the halogens tend to form anions because a. 2) have 7 valence electrons and need one more. B).
PPT Group 17 The Halogens PowerPoint Presentation, free download
1) they give up one electron to achieve the octet rule. Why does halogen tend to form an anion? B) gaining electrons will fill their octet faster than losing them. Web se cr ar ar the halogens tend to form anions because a. B) gaining electrons will make them attain a.
Which of the Following Exists as a Polyatomic Molecule
Web halogens tend to form anions because a) losing electrons will fill their octet faster than gaining them. B) gaining electrons will fill. Why does halogen tend to form an anion? The halogens do exist in the +1 oxidation state, for example, in oclx− o c l x −. C) anions are usually larger than their corresponding atom.
Halogens tend to form anions because A) losing
B) gaining electrons will fill. To begin with, halogens are those elements found in group 17 ( fomerly group 7) of the periodic table and they include; The stoichiometry and route of. Why does halogen tend to form an anion? Well, your question might actually require a direct answer but let me try expounding it in a better understandable way.
PPT Chapter 22 Chemistry of the Nonmetals PowerPoint Presentation
Option b halogens have 7. The middle halogens—chlorine, bromine, and. B) gaining electrons will fill. Well, your question might actually require a direct answer but let me try expounding it in a better understandable way. Web halogens tend to form anions because a) losing electrons will fill their octet faster than gaining them.
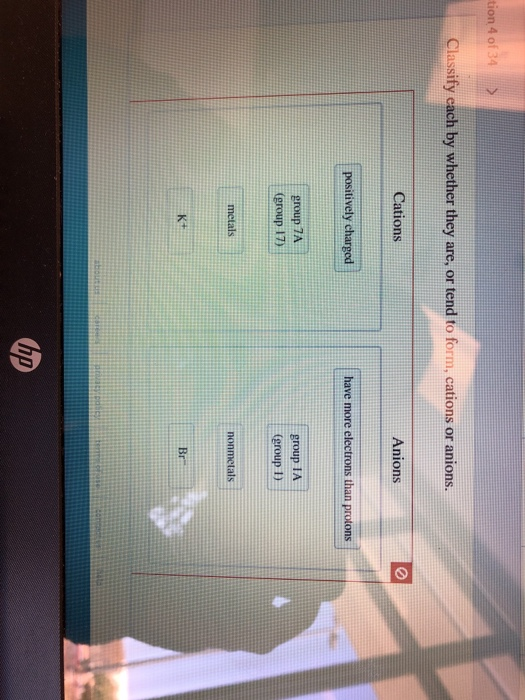
Solved tion 4 of 34 Classify each by whether they are, or
It is a subtle matter. Halogens have 7 electrons in their valence shell, so they tend to gain 1 electron to fill their octet. Well, your question might actually require a direct answer but let me try expounding it in a better understandable way. Web + anions + chemistry. The middle halogens—chlorine, bromine, and.
Constants I Periodic Table Learning Goal To familiar with the
2) have 7 valence electrons and need one more. Well, your question might actually require a direct answer but let me try expounding it in a better understandable way. Web generally speaking the elements in the top right side of the periodic table (the nonmetals and halogens) tend to form anions. Option b halogens have 7. To begin with, halogens.
Cations And Anions List Tips to memorize cations YouTube
Web 68) a) atoms are usually larger than their corresponding cation b) the halogens tend to form 1+ ions. Web all of the halogens form acids when bonded to hydrogen. B) gaining electrons will make them attain a. B) gaining electrons will fill their octet faster than losing them. The halogens do exist in the +1 oxidation state, for example,.
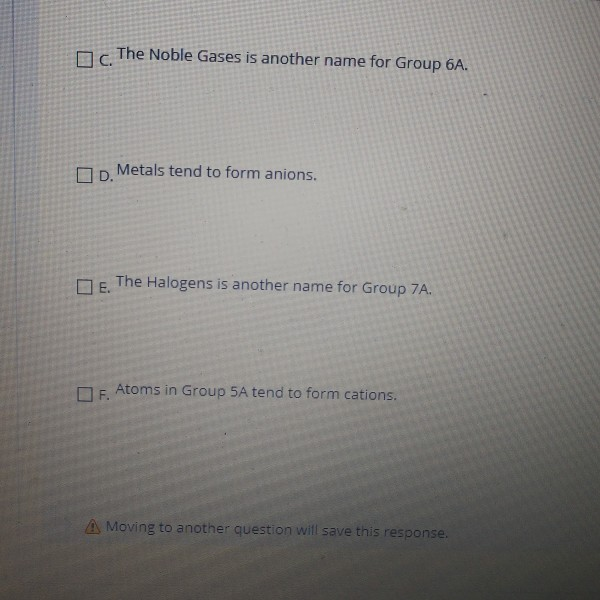
Solved Question 32 Which of the following statements about
Web generally speaking the elements in the top right side of the periodic table (the nonmetals and halogens) tend to form anions. Web all of the halogens form acids when bonded to hydrogen. Web c) halogen element ionization energies are lower than transition metal ionization energies. The halogens do exist in the +1 oxidation state, for example, in oclx− o.
Solved 13Atoms Of The Elements N And P Have Athe Same
Option b halogens have 7. Web halogens (flourine, chlorine, bromine, iodine, astatine) and oxygen tend to form anions when bonding with other elements. The middle halogens—chlorine, bromine, and. Web answered • expert verified. B) gaining electrons will fill their octet faster than losing them.
The Halogens Do Exist In The +1 Oxidation State, For Example, In Oclx− O C L X −.
Web + anions + chemistry. Web answered • expert verified. B) gaining electrons will fill their octet faster than losing them. Web the halogens tend to form 1+ ions because they only need one electron to fill their valence shell.
Why Does Halogen Tend To Form An Anion?
Web generally speaking the elements in the top right side of the periodic table (the nonmetals and halogens) tend to form anions. The halogens tend to form anions because. B) gaining electrons will make them attain a. The middle halogens—chlorine, bromine, and.
Web Halogens Tend To Form Anions Because A) Losing Electrons Will Fill Their Octet Faster Than Gaining Them.
Web halogens tend to form anions because a) losing electrons will make them attain a noble gas configuration faster than gaining them. It is a subtle matter. Web all of the halogens form acids when bonded to hydrogen. They have low electron affinities c.
B) Gaining Electrons Will Fill Their Octet Faster Than Losing Them.
Halogens tend to form anions because a) losing electrons will fill their octet faster than gaining them. D) halogen atomic radii tend to be larger than their corresponding ionic radii. They have low first ionization energies b. The stoichiometry and route of.