Iron Electron Configuration Long Form
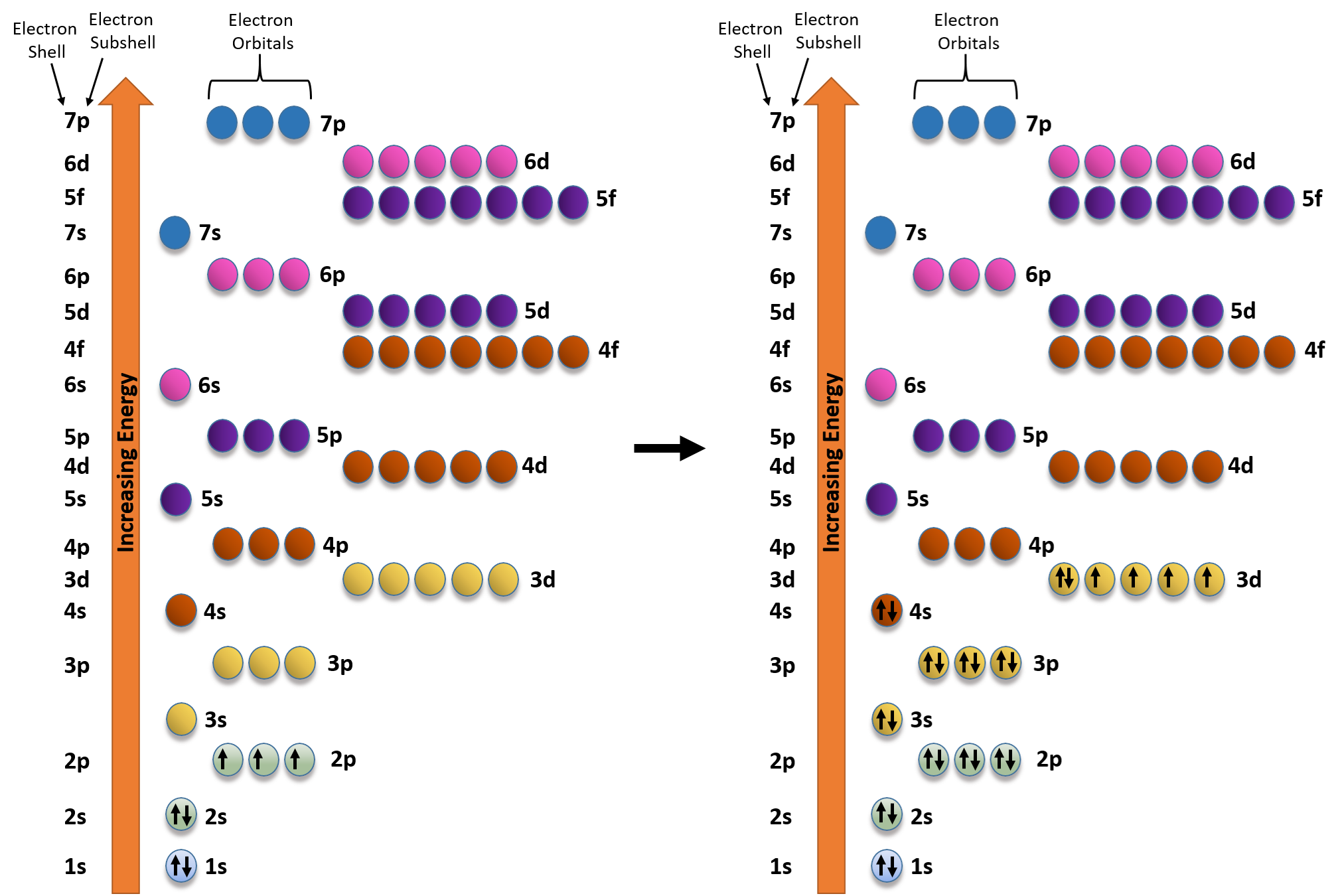
Iron Electron Configuration Long Form - Fe 1s22s22p63s23p64s23d6 when we make a 3+ ion for iron, we need to take the electrons from the outermost shell first so that would be the 4s shell not the 3d shell: Web answer (1 of 3): And most of the rest stored in the body (. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. Web 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6 to write an electron configuration for any element you'll need to apply the aufbau principle and use a diagonal diagram. Electron configuration of fluorine (f) [he] 2s 2 2p 5: Its core orbitals are the 1s, 2s, 2p 's, 3s, and 3p 's. Its valence orbitals are the 4s and 3d 's. Web in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe.
Fe 1s22s22p63s23p64s23d6 when we make a 3+ ion for iron, we need to take the electrons from the outermost shell first so that would be the 4s shell not the 3d shell: 1s 2 2s 2 2p 3: When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. The atomic number of iron is 26. Electron configuration of fluorine (f) [he] 2s 2 2p 5: Both are explained in the video below. Web electronic iron configuration will be : For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in. Web the average quantity of iron in the human body is about 4.5 grams (about 0.004 percent), of which approximately 65 percent is in the form of hemoglobin, which transports molecular oxygen from the lungs throughout the body; 1 percent in the various enzymes that control intracellular oxidation;
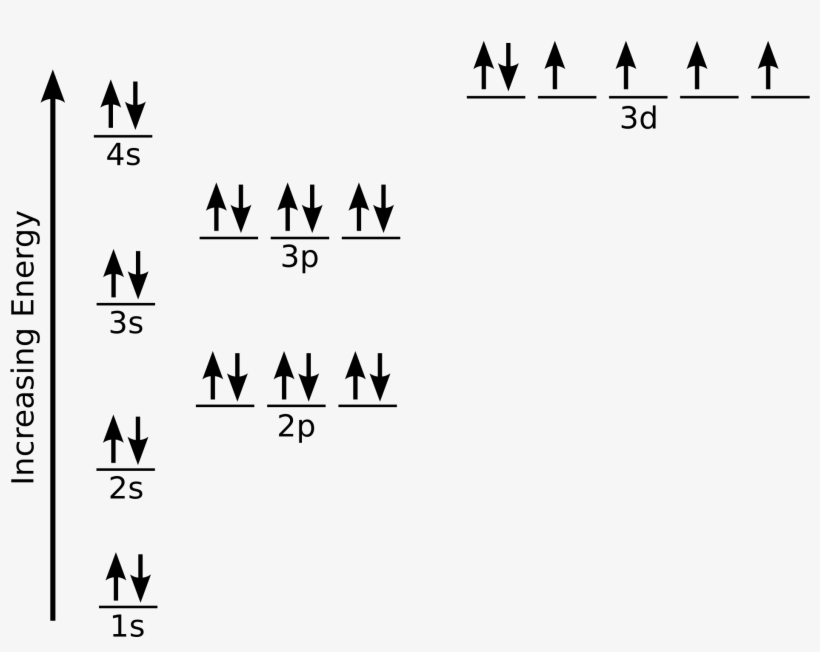
Web the electronic iron configuration (e.g.) is 1s2 2s2 2p6 3s2 3p6 4s2 3d6, and the form [ ar ] 4s2 3d6 is abbreviated. And most of the rest stored in the body (. Applys lean manufacturing principles to metal fabrication. Web what we have is: Electron configuration of oxygen (o) [he] 2s 2 2p 4: Both are explained in the video below. 1s 2 2s 2 2p 6: Web iron has 26 electrons so its normal electron configuration would be: Web the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. Web electron configuration of nitrogen (n) [he] 2s 2 2p 3:
Iron Periodic Table Electrons Review Home Decor
Electron configuration of fluorine (f) [he] 2s 2 2p 5: Electron configurations describe where electrons are located around the nucleus of an atom. Electron configuration of neon (ne) [he] 2s 2 2p 6: Its valence orbitals are the 4s and 3d 's. Web answer (1 of 3):
Iron Periodic Table Electrons Awesome Home
1s 2 2s 2 2p 3: Iron, a chemical substance or element, is a metal generally represented by its symbol fe. Web commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating with.
The Electron 2020 Give The Electron Configuration Of Chlorine
Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. The atomic number of iron is 26. Fe3+1s22s22p63s23p63d5 one other note on writing electron configurations: [ar]3d 6 4s 2 long form: The element symbol, fe, was shortened from the latin word ' ferrum ' meaning 'firmness'.
Electron Configuration For Iron Iii Ion Rapid Electron
We describe an electron configuration with a symbol that contains three pieces of information ( figure 6.25 ): Of the elements discussed in the video, iron is most like sc, but iron has 5 more electrons in the last subshell which is filled with electrons making its. Electron configuration of neon (ne) [he] 2s 2 2p 6: Web as an.
Open Iron Electron Configuration Free Transparent PNG Download PNGkey
1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2 shell structure: Web electron configuration the arrangements of electrons above the last (closed shell) noble gas. Web its 26 electrons are arranged in the configuration [ar]3d 6 4s 2, of which the 3d and 4s electrons are relatively close in energy, and thus a number of.
Electron Configurations Long Form YouTube
Web overview test series the arrangement of electrons in the orbital shells surrounding the nucleus is known as an atom's electronic configuration. Web iron electron configuration short form: The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. 1s 2 2s 2 2p 6 3s 2 3p 6 3d.
Electron Configuration Of Iron Full worksheet today
Web commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating with the loss of or gain of electrons in their subsequent orbitals. Fe3+1s22s22p63s23p63d5 one other note on writing electron configurations: Both are.
Write the full electron configuration of iron, YouTube
Web the electronic iron configuration (e.g.) is 1s2 2s2 2p6 3s2 3p6 4s2 3d6, and the form [ ar ] 4s2 3d6 is abbreviated. Its symbol is short for ferrum which is a latin term. Web commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to.
【5 Steps】Electron Configuration of Iron(Fe) Electron configuration
1s 2 2s 2 2p 6: Web iron has 26 electrons so its normal electron configuration would be: Web what we have is: When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the iron atom. Iron is a member of group 8 and the first transition series of the periodic.
39+ Electron Configuration Of Iron Unabbreviated mariamirandag
Web the commonly used long form of the periodic table is designed to emphasize electron configurations. The electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. Iron can take on many forms, including cast, wrought, and pig iron, but suffers badly from rust if not protected in some way..
\[1S^{2} 2S^{2} 2P^{6} 3S^{2} 3P^{6} 4S^{2} 3D^{6}\].
Web electron configuration of nitrogen (n) [he] 2s 2 2p 3: This knowledge is essential for comprehending an element's chemical and physical characteristics, such as its reactivity, magnetic properties, spectral characteristics, and position in the periodic table. 1s 2 2s 2 2p 6: Its symbol is short for ferrum which is a latin term.
Web Overview Test Series The Arrangement Of Electrons In The Orbital Shells Surrounding The Nucleus Is Known As An Atom's Electronic Configuration.
Electron configuration of oxygen (o) [he] 2s 2 2p 4: 1 percent in the various enzymes that control intracellular oxidation; Its core orbitals are the 1s, 2s, 2p 's, 3s, and 3p 's. Web as an approximate rule, electron configurations are given by the aufbau principle and the madelung rule.
Web Iron Electron Configuration Short Form:
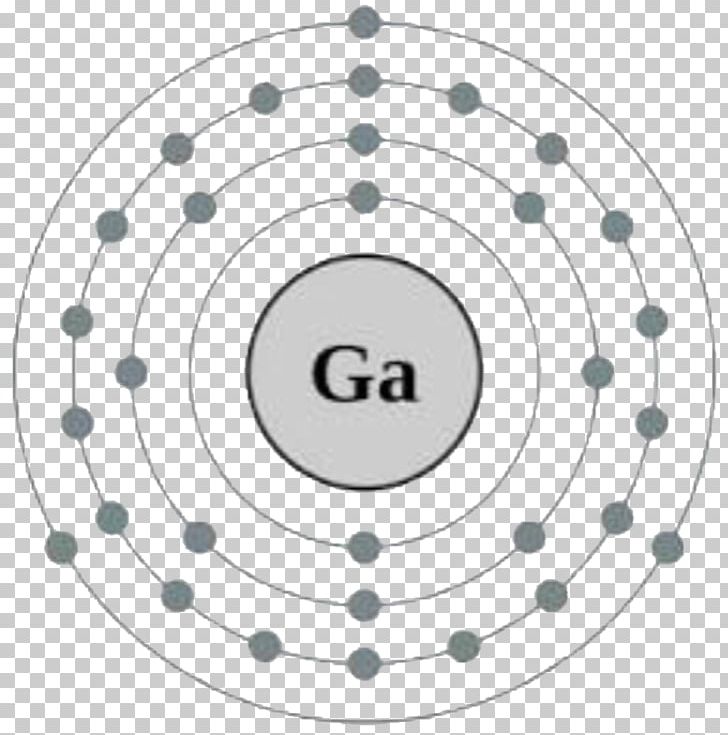
Web its 26 electrons are arranged in the configuration [ar]3d 6 4s 2, of which the 3d and 4s electrons are relatively close in energy, and thus a number of electrons can be ionized. 2 8 14 2 iron discovery discovery date: Electron configuration of fluorine (f) [he] 2s 2 2p 5: The number of the principal quantum shell, n, the letter that designates the orbital type (the subshell, l ), and
Offers The Shortest Lead Times At The Lowest Variable Cost, With Flexibility To Accommodate Volume Fluctuations And Part.
Nonetheless, it wasn’t easy to find the reason why it was written as [ ar ] 3d6 4s2 instead in some periodic table. [ar]3d 6 4s 2 long form: Iron can take on many forms, including cast, wrought, and pig iron, but suffers badly from rust if not protected in some way. Both are explained in the video below.