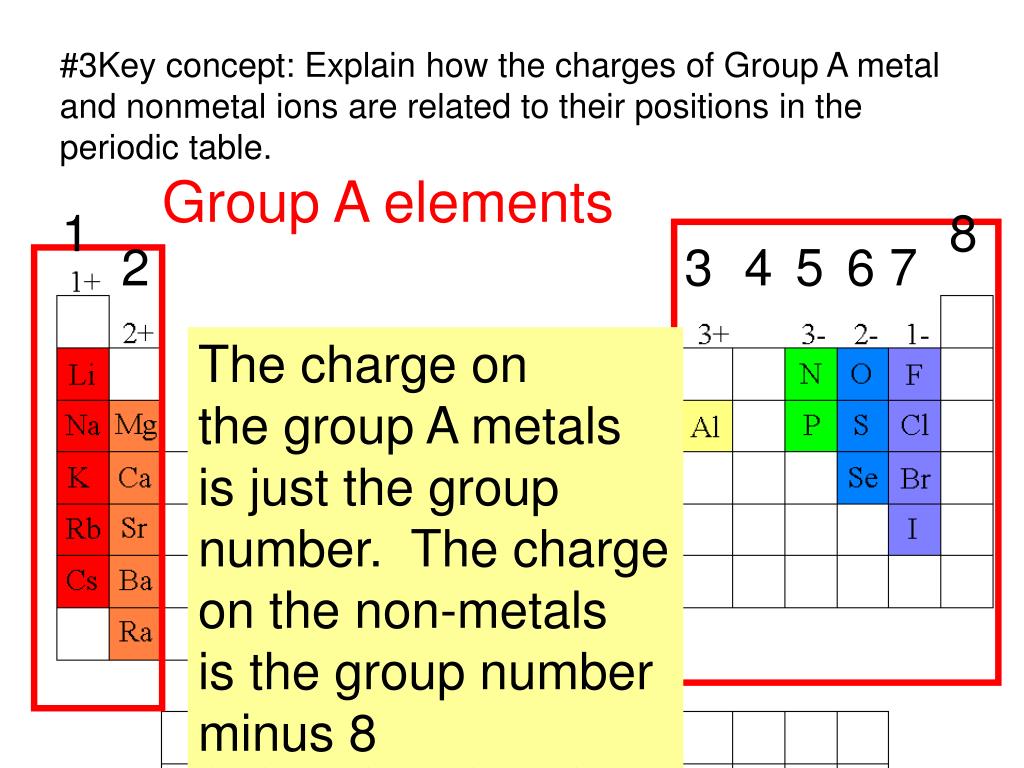
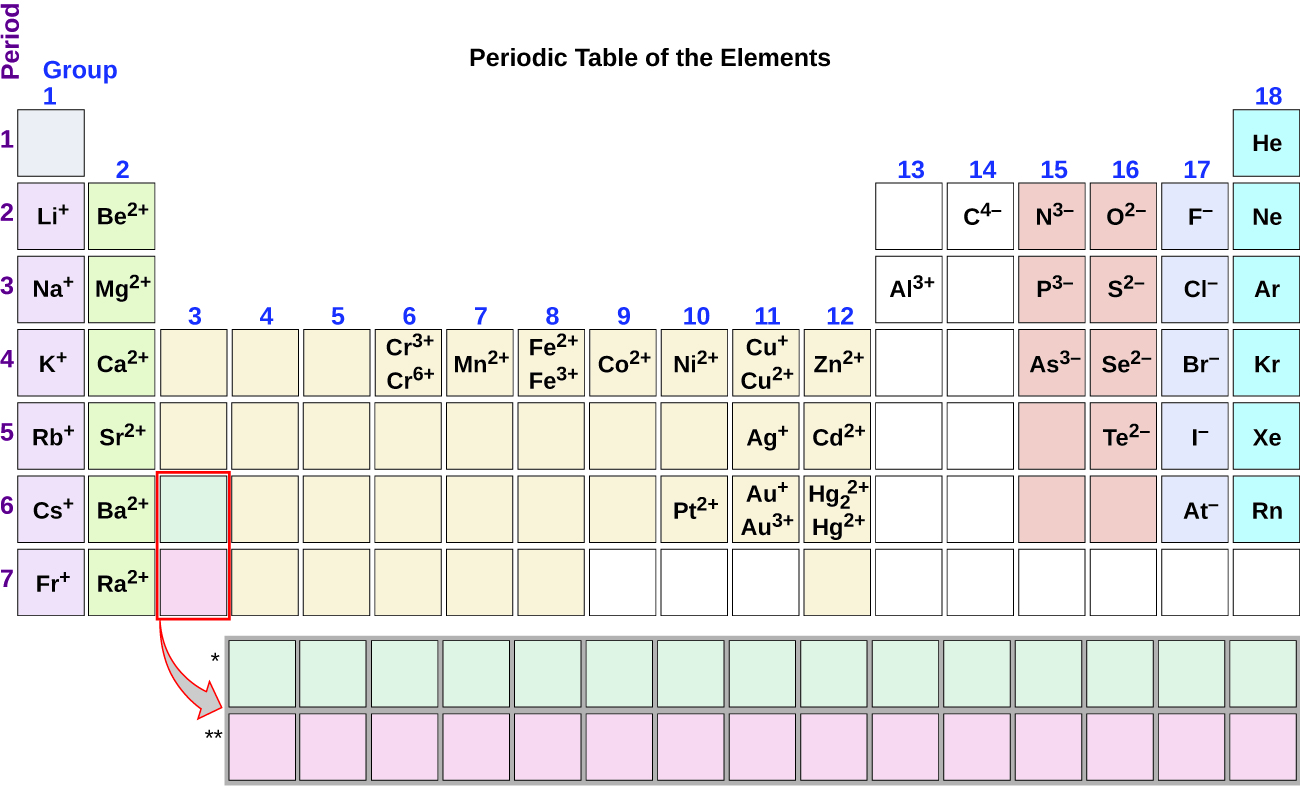
Metals That Form One Ion
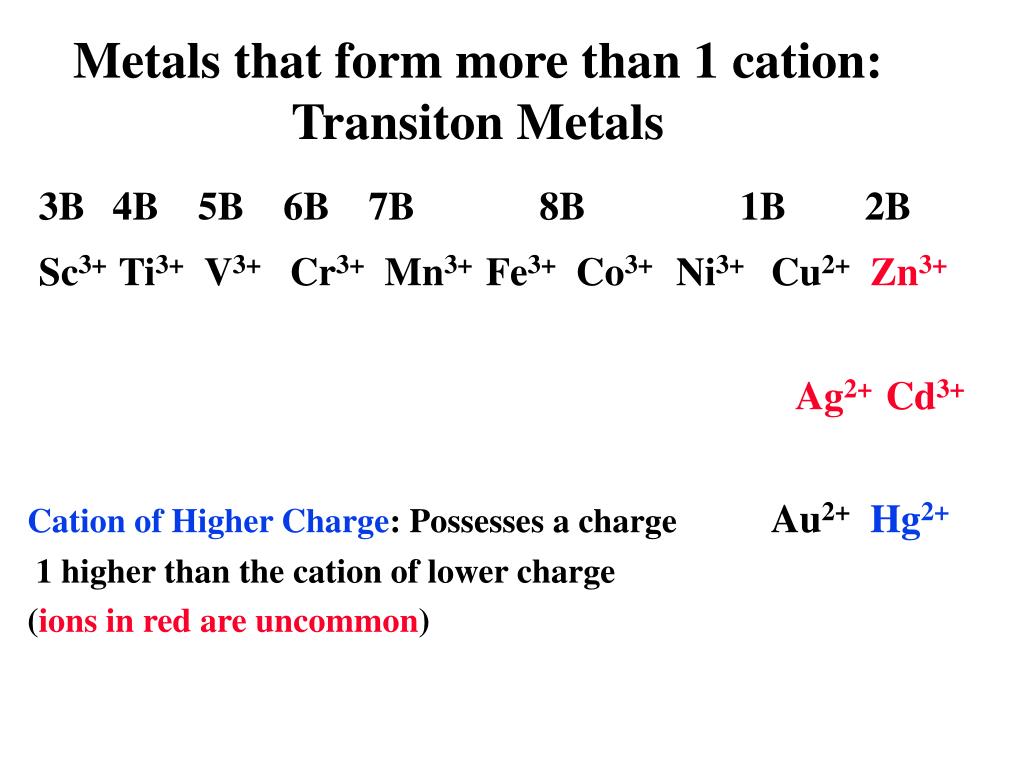
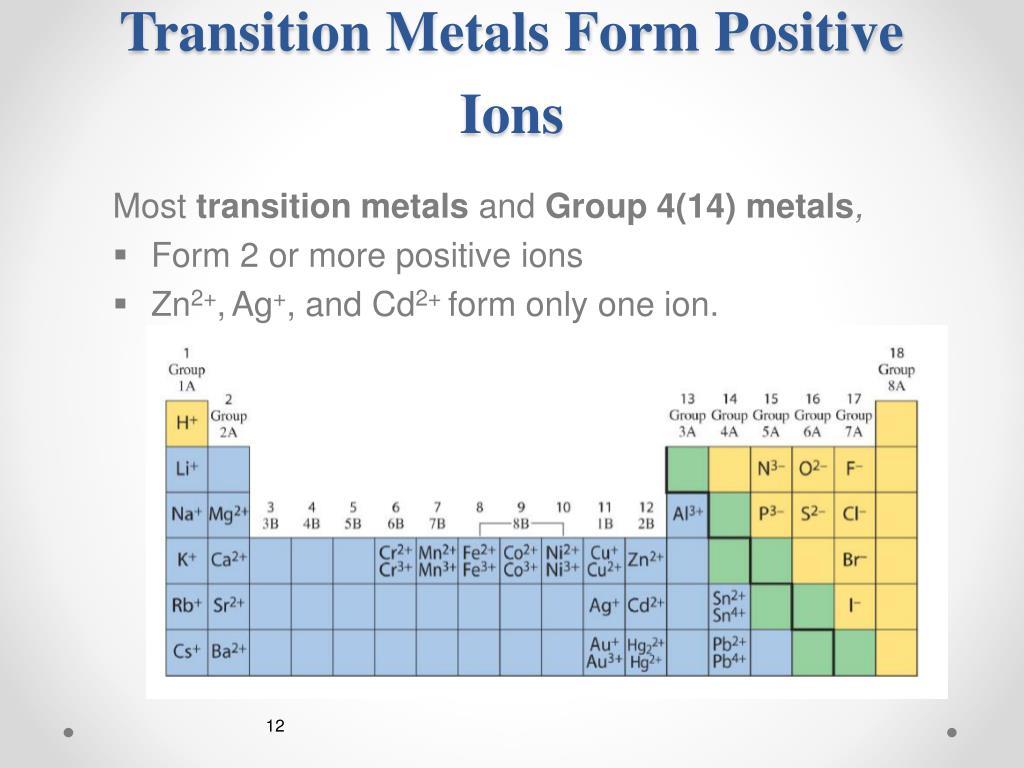
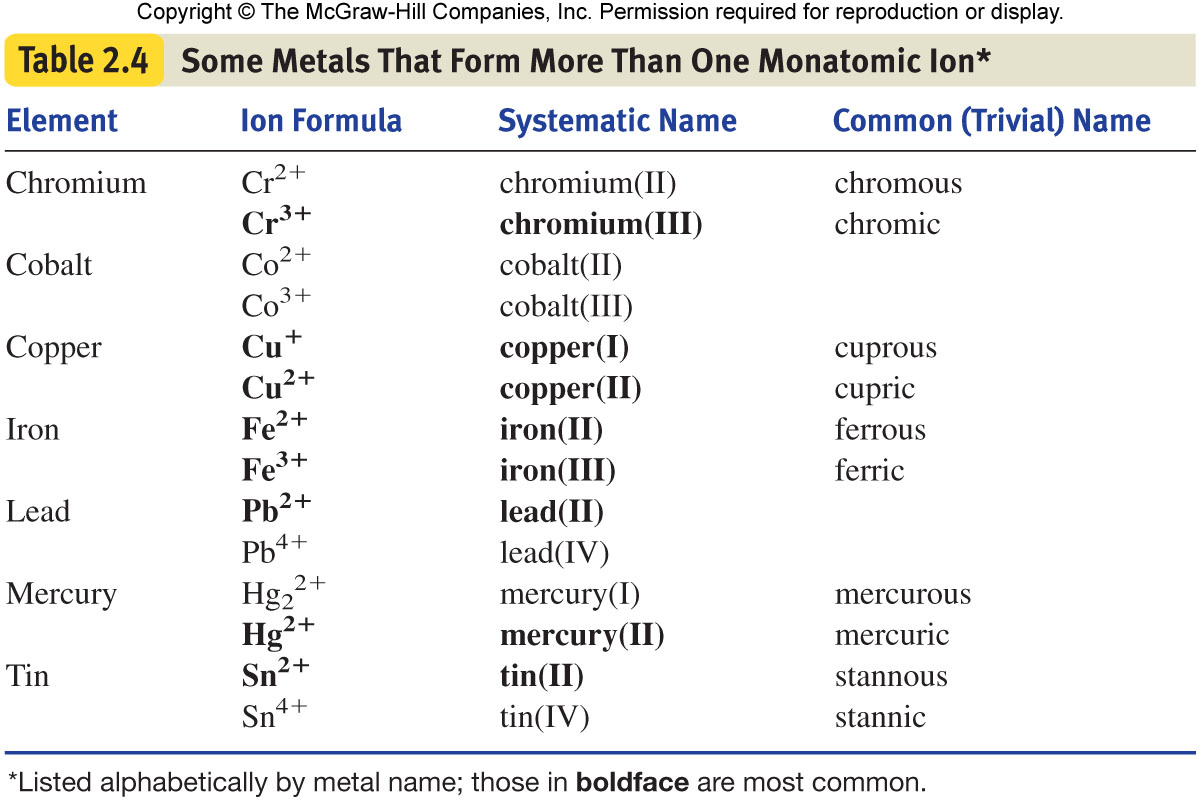
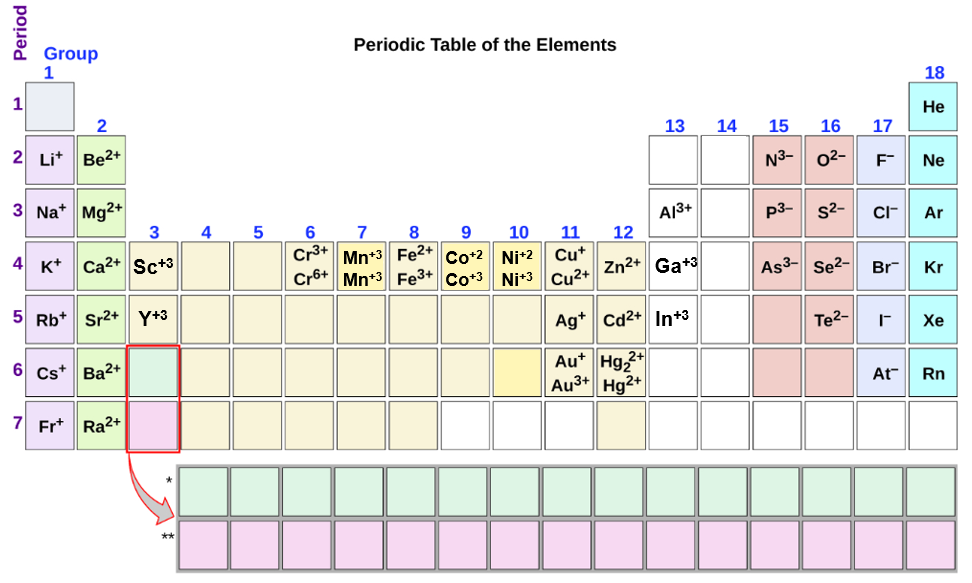
Metals That Form One Ion - Metals tend to form cations, while nonmetals tend to form anions. Web transition metals can exist in more than one stable oxidation state; Web 9 rows metals that form more than one ion ion stock name ion stock name polyatomic ions ion. Second, most atoms form ions of a. Thus, na + is the sodium ion, al 3+ is the aluminum. Element ion classical name stock name chromium cr2+ chromous ion chromium (ii) ion cr3+ chromic ion chromium (iii) ion. Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Web a metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m(h 2 o) n] z+.the solvation number, n, determined by a variety of. As shown in figure 5.1 (a) , many metals can form more than one cation. Web this is actually one of the chemical properties of metals and nonmetals:
Web predict whether the following compounds are ionic or molecular: As shown in figure 5.1 (a) , many metals can form more than one cation. An ion is an atom or group of atoms with a positive or negative charge. Web a metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m(h 2 o) n] z+.the solvation number, n, determined by a variety of. Second, most atoms form ions of a. These are generally transition metal ions like copper or iron. There is no space between. Second, elements that live in the first. Click the card to flip 👆. The name of a monatomic cation is simply the name of the element followed by the word ion.
In other words, they can form different ions. These are generally transition metal ions like copper or iron. Thus, na + is the sodium ion, al 3+ is the aluminum. There is no space between. Web 9 rows metals that form more than one ion ion stock name ion stock name polyatomic ions ion. Metals that form more than one cation. Positively charged ions are called cations; Web metals that form only one cation. Web metals that form more than one cation. Second, elements that live in the first.
PPT The Nomenclature of Binary Compounds PowerPoint Presentation ID
Web predict whether the following compounds are ionic or molecular: Web finally we need to address the compounds we can make using elements that can form more than one ion. These are generally transition metal ions like copper or iron. Element ion classical name stock name chromium cr2+ chromous ion chromium (ii) ion cr3+ chromic ion chromium (iii) ion. Ki,.
Naming Simple Ionic Compounds Pathways to Chemistry
H 2 o 2, the bleach and disinfectant. Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges. These are generally transition metal ions like copper or iron. In these cases, chemists do not use a roman. There is no space between.
PPT Chapter 4 Compounds and Their Bonds PowerPoint Presentation, free
Web this is actually one of the chemical properties of metals and nonmetals: Web finally we need to address the compounds we can make using elements that can form more than one ion. Ki, the compound used as a source of iodine in table salt. Web a metal ion in aqueous solution or aqua ion is a cation, dissolved in.
PPT Naming Ionic Compounds PowerPoint Presentation, free download
An ion is an atom or group of atoms with a positive or negative charge. Metals tend to form cations, while nonmetals tend to form anions. Web metals that form only one cation. Element ion classical name stock name chromium cr2+ chromous ion chromium (ii) ion cr3+ chromic ion chromium (iii) ion. Web predict whether the following compounds are ionic.
Metal Ions in Life Sciences Set Volumes 8 9 価格 河合nl/mのブログ
Ki, the compound used as a source of iodine in table salt. Web metals that form only one cation. Web predict whether the following compounds are ionic or molecular: In other words, they can form different ions. Thus, na + is the sodium ion, al 3+ is the aluminum.
2.6 Ionic Compounds and Formulas Chemistry LibreTexts
The name of a monatomic cation is simply the name of the element followed by the word ion. Positively charged ions are called cations; As shown in figure 5.1 (a) , many metals can form more than one cation. Web 9 rows metals that form more than one ion ion stock name ion stock name polyatomic ions ion. Web metals.
PPT 1 Name the ions formed by these elements and classify them as
Web a metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m(h 2 o) n] z+.the solvation number, n, determined by a variety of. Web predict whether the following compounds are ionic or molecular: In other words, they can form different ions. Web a roman numeral in parentheses, followed by the name.
2.6 Ionic Compounds and Formulas Chemistry LibreTexts
Web metals that form more than one cation. These are generally transition metal ions like copper or iron. The charge of an electron is considered to be negative by convention and this charge is equal and. Click the card to flip 👆. Web 9 rows metals that form more than one ion ion stock name ion stock name polyatomic ions.
4.1.5 State that transition elements can form more than one ion IB
These are generally transition metal ions like copper or iron. Second, elements that live in the first. The name of a monatomic cation is simply the name of the element followed by the word ion. There is no space between. Web this is actually one of the chemical properties of metals and nonmetals:
PPT 1 Name the ions formed by these elements and classify them as
Thus, na + is the sodium ion, al 3+ is the aluminum. Web this is actually one of the chemical properties of metals and nonmetals: Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Click the card to flip 👆. Web metals that form only one cation.
Metals Tend To Form Cations, While Nonmetals Tend To Form Anions.
Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Web sometimes transition metals form only one ion, such as silver, which forms ag zinc, which forms zn and cadmium, which forms cd. An ion is an atom or group of atoms with a positive or negative charge. Web metals that form only one cation.
Web Cations Of Metals That Form Only One Type Of Ion.
Thus, na + is the sodium ion, al 3+ is the aluminum. Web a metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m(h 2 o) n] z+.the solvation number, n, determined by a variety of. H 2 o 2, the bleach and disinfectant. The name of the cation of a metal that.
The Charge Of An Electron Is Considered To Be Negative By Convention And This Charge Is Equal And.
In other words, they can form different ions. Web metals that form more than one cation. Web transition metals can exist in more than one stable oxidation state; Web predict whether the following compounds are ionic or molecular:
In These Cases, Chemists Do Not Use A Roman.
Web a roman numeral in parentheses, followed by the name of the element, is used for elements that can form more than one positive ion. Second, elements that live in the first. As shown in figure 5.1 (a) , many metals can form more than one cation. Click the card to flip 👆.