Olumiant Enrollment Form Alopecia
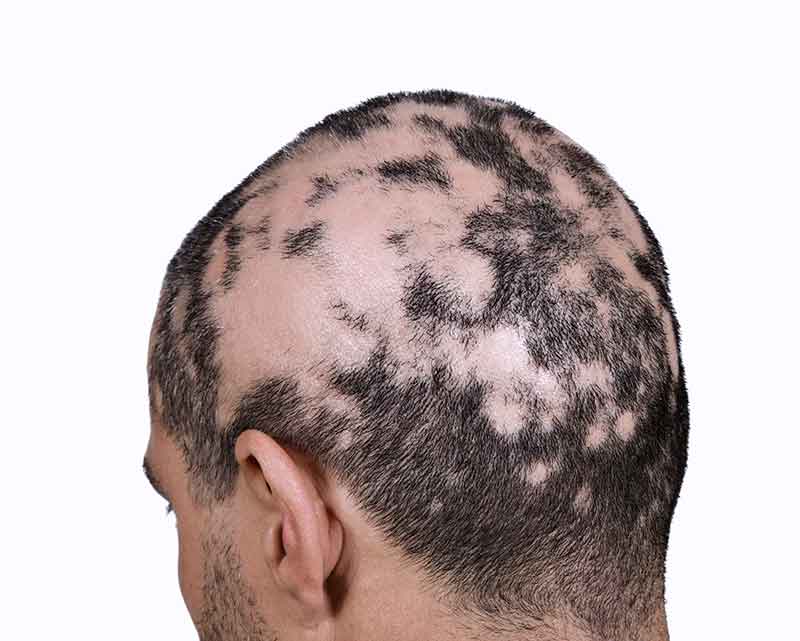
Olumiant Enrollment Form Alopecia - Web alopecia areata causes hair loss that can happen unpredictably. Food and drug administration announced its approval of eli lilly's oral tablet olumiant (baricitinib). Hair loss can affect your scalp or any place where hair grows on your body. Learn about its dosage, form, strengths, and more. Enroll in olumiant together™ and you may be eligible to save on the cost of olumiant. Web olumiant (baricitinib) is a janus kinase (jak) inhibitor indicated for the treatment of adult patients with moderately to severely active rheumatoid arthritis who have had an. Food and drug administration (fda). Permanent & protected by the bosley® guarantee.* learn more with a free info kit. Enroll in olumiant together™ and you may be eligible to save on the cost of olumiant. Web the drug is sold by eli lilly & co.
Web olumiant (baricitinib) is a new treatment for hair loss due to severe alopecia areata, a condition in which the immune system attacks hair follicles. Web the food and drug administration (fda) has approved the drug olumiant (baricitinib), made by eli lilly, as the first oral treatment of severe alopecia areata. Web olumiant is indicated for the treatment of adult patients with severe alopecia areata. Web olumiant (baricitinib) is an oral janus kinase (jak) inhibitor approved by the u.s. Ad view litfulo™ prescribing info, safety info & boxed warning on the official hcp site. Download support resources, including a doctor discussion guide. Ad view prescribing info, safety info & boxed warning. Permanent & protected by the bosley® guarantee.* learn more with a free info kit. Official patient site for litfulo™. Enroll in olumiant together™ and you may be eligible to save on the cost of olumiant.
Web olumiant is indicated for the treatment of adult patients with severe alopecia areata. Web the food and drug administration (fda) has approved the drug olumiant (baricitinib), made by eli lilly, as the first oral treatment of severe alopecia areata. Enroll in olumiant together™ and you may be eligible to save on the cost of olumiant. Web impairment (estimated glomerular filtration rate (gfr) between 30 and olumiant</strong> is not recommended. Not recommended for use in combination with other jak inhibitors,. Official patient site for litfulo™. Ad schedule a free consultation with one of our hair restoration experts today! Download support resources, including a doctor discussion guide. Enroll in olumiant together™ and you may be eligible to save on the cost of olumiant. Food and drug administration (fda) for adults with severe alopecia areata (aa) (1).
Alopecia Areata Avens Blog Avens Blog
Ad view litfulo™ prescribing info, safety info & boxed warning on the official hcp site. Permanent & protected by the bosley® guarantee.* learn more with a free info kit. Enroll in olumiant together™ and you may be eligible to save on the cost of olumiant. Today, history was made as the u.s. Ad view efficacy, safety, full prescribing information, and.
Alopecia Areata Treated With Tofacinitib Hair Disorders JAMA
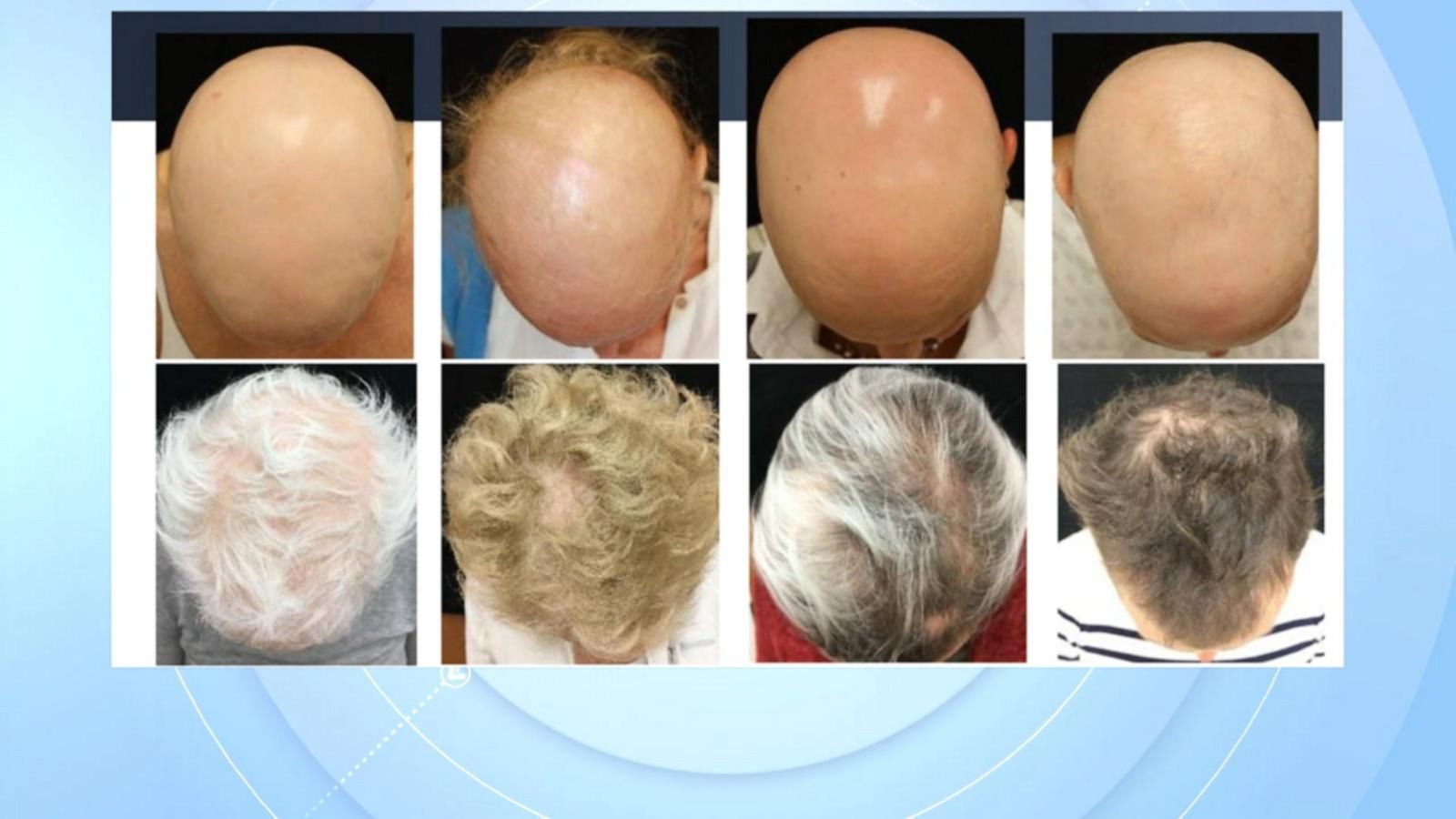
Official patient site for litfulo™. Web “the results of clinical trials are remarkable, as 1 in 5 adults taking olumiant 2mg/day and 1 in 3 taking olumiant 4mg/day achieved significant hair regrowth resulting. Learn about its dosage, form, strengths, and more. Hair loss can affect your scalp or any place where hair grows on your body. Web olumiant (baricitinib) is.
Olumiant es designada Terapia Innovadora por la FDA para el tratamiento
Food and drug administration (fda). Learn about its dosage, form, strengths, and more. Ad view litfulo™ prescribing info, safety info & boxed warning on the official hcp site. Ad view efficacy, safety, full prescribing information, and boxed warning. Official patient site for litfulo™.
Alopecia Universalis Successfully Treated With Adalimumab Hair
Web olumiant (baricitinib) is an oral janus kinase (jak) inhibitor approved by the u.s. Web olumiant (baricitinib) is a janus kinase (jak) inhibitor indicated for the treatment of adult patients with moderately to severely active rheumatoid arthritis who have had an. Hair loss can affect your scalp or any place where hair grows on your body. Permanent & protected by.
Another ‘miracle drug’ for COVID19 increasingly hard to find in
Web olumiant is indicated for the treatment of adult patients with severe alopecia areata. Not recommended for use in combination with other jak inhibitors,. Web olumiant is indicated for the treatment of adult patients with severe alopecia areata. Food and drug administration (fda) for adults with severe alopecia areata (aa) (1). Enroll in olumiant together™ and you may be eligible.
FDA Approves Eli Lilly's Drug Olumiant For Alopecia Dermatology
Web fda approves olumiant™ (baricitinib) for adults with severe alopecia areata. Web “the results of clinical trials are remarkable, as 1 in 5 adults taking olumiant 2mg/day and 1 in 3 taking olumiant 4mg/day achieved significant hair regrowth resulting. Ad schedule a free consultation with one of our hair restoration experts today! Find resources and support for your patients prescribed.
Olumiant offers hope to patients with rheumatoid arthritis Drug
Permanent & protected by the bosley® guarantee.* learn more with a free info kit. Enroll in olumiant together™ and you may be eligible to save on the cost of olumiant. Ad view efficacy, safety, full prescribing information, and boxed warning. Ad schedule a free consultation with one of our hair restoration experts today! Not recommended for use in combination with.
FDA approves Olumiant to treat severe cases of alopecia areata
Food and drug administration (fda) for adults with severe alopecia areata (aa) (1). Web olumiant is indicated for the treatment of adult patients with severe alopecia areata. Web the drug is sold by eli lilly & co. Web olumiant (baricitinib) is a new treatment for hair loss due to severe alopecia areata, a condition in which the immune system attacks.
FDA approves use of Olumiant to help treat severe alopecia areata
Web impairment (estimated glomerular filtration rate (gfr) between 30 and olumiant</strong> is not recommended. Find resources and support for your patients prescribed litfulo®. Web olumiant (baricitinib) is a janus kinase (jak) inhibitor indicated for the treatment of adult patients with moderately to severely active rheumatoid arthritis who have had an. Web fda approves olumiant™ (baricitinib) for adults with severe alopecia.
Dosing for Rheumatoid Arthritis HCP Olumiant® (baricitinib)
Web the drug is sold by eli lilly & co. Food and drug administration approved olumiant (baricitinib) oral tablets to treat adult patients with severe alopecia areata, a disorder that. Ad view efficacy, safety, full prescribing information, and boxed warning. Enroll in olumiant together™ and you may be eligible to save on the cost of olumiant. Web olumiant (baricitinib) is.
Not Recommended For Use In Combination With Other Jak Inhibitors,.
Enroll in olumiant together™ and you may be eligible to save on the cost of olumiant. Food and drug administration (fda) for adults with severe alopecia areata (aa) (1). Web olumiant (baricitinib) is a new treatment for hair loss due to severe alopecia areata, a condition in which the immune system attacks hair follicles. Web olumiant (baricitinib) is a janus kinase (jak) inhibitor indicated for the treatment of adult patients with moderately to severely active rheumatoid arthritis who have had an.
Web Olumiant (Baricitinib) Is An Oral Janus Kinase (Jak) Inhibitor Approved By The U.s.
Find resources and support for your patients prescribed litfulo®. Food and drug administration (fda). Web june 13, 2022 español today, the u.s. Official patient site for litfulo™.
Download Support Resources, Including A Doctor Discussion Guide.
Web the food and drug administration (fda) has approved the drug olumiant (baricitinib), made by eli lilly, as the first oral treatment of severe alopecia areata. Ad view efficacy, safety, full prescribing information, and boxed warning. Permanent & protected by the bosley® guarantee.* learn more with a free info kit. Enroll in olumiant together™ and you may be eligible to save on the cost of olumiant.
Web Fda Approves Olumiant™ (Baricitinib) For Adults With Severe Alopecia Areata.
Web “the results of clinical trials are remarkable, as 1 in 5 adults taking olumiant 2mg/day and 1 in 3 taking olumiant 4mg/day achieved significant hair regrowth resulting. But some current treatments (though not. Hair loss can affect your scalp or any place where hair grows on your body. Ad view prescribing info, safety info & boxed warning.