Sodium Oxide Combines With Water To Form Sodium Hydroxide
Sodium Oxide Combines With Water To Form Sodium Hydroxide - Na2o + h2o → 2nah + o2. Asked • 08/03/15 the balanced equation for the reaction between sodium oxide and water is a. Web yes it is a hydroxide for a word equasion it is sodium + water = sodium hydroxide + hydrogen what is sodium oxide combines with water to make sodium. Web can you please balance this equation for me: Web who balanced equation for to reaction between sodium oxide and water is. Na2o + h2o → 2naoh b. Web expert answer transcribed image text: Reaction with alcohols gives their. Na2o + h2o → 2nah + o2 c. Web sodium oxide + water → sodium hydroxide [na2o + h2o → 2 naoh] is an example of get the answers you need, now!
Web can you please balance this equation for me: How many grams of sodium. The balanced chemical equation for sodium oxide. Web who balanced equation for to reaction between sodium oxide and water is. Web generally, elemental sodium is more reactive than lithium, and it reacts with water to form a strong base, sodium hydroxide (naoh). Na+h2o>naoh that is sodium reacting with water to give sodium hydroxide. Sodium oxide reacts with water to produce sodium hydroxide. Sodium oxide reacts exothermically with cold water to produce sodium hydroxide solution. Na 2 o + h 2 o → 2 naoh. Na2o + h2o → 2naoh b.
Na2o + h2o → 2naoh b. Na2o + h2o → 2nah + o2. Web solution reaction of sodium oxide with water: Na2o + h2o → 2na +. Na2o + h2o → 2naoh b. Sodium oxide reacts with water to produce sodium hydroxide. Web can you please balance this equation for me: Web sodium oxide + water → sodium hydroxide [na2o + h2o → 2 naoh] is an example of get the answers you need, now! How many grams of sodium. Reaction with alcohols gives their.
Preparation of 10 M Sodium Hydroxide (NaOH) Solution Laboratory Notes
Na2o + h2o → 2na +. Web generally, elemental sodium is more reactive than lithium, and it reacts with water to form a strong base, sodium hydroxide (naoh). Web yes it is a hydroxide for a word equasion it is sodium + water = sodium hydroxide + hydrogen what is sodium oxide combines with water to make sodium. Depending on.
SODIUM HYDROXIDE, 1.0N 500ML H
Web science chemistry chemistry questions and answers write the word equation and a balanced chemical equation for each reaction below. Na2o + h2o → 2nah + o2 c. Reaction with alcohols gives their. Web who balanced equation for to reaction between sodium oxide and water is. First of all, the equation.
PPT Balancing Equations ANSWER KEY PowerPoint Presentation ID2276630
Its chemistry is well explored. The balanced chemical equation for sodium oxide. Web sodium oxide + water → sodium hydroxide [na2o + h2o → 2 naoh] is an example of get the answers you need, now! Na2o + h2o → 2nah + o2 c. Na 2 o + h 2 o → 2 naoh.
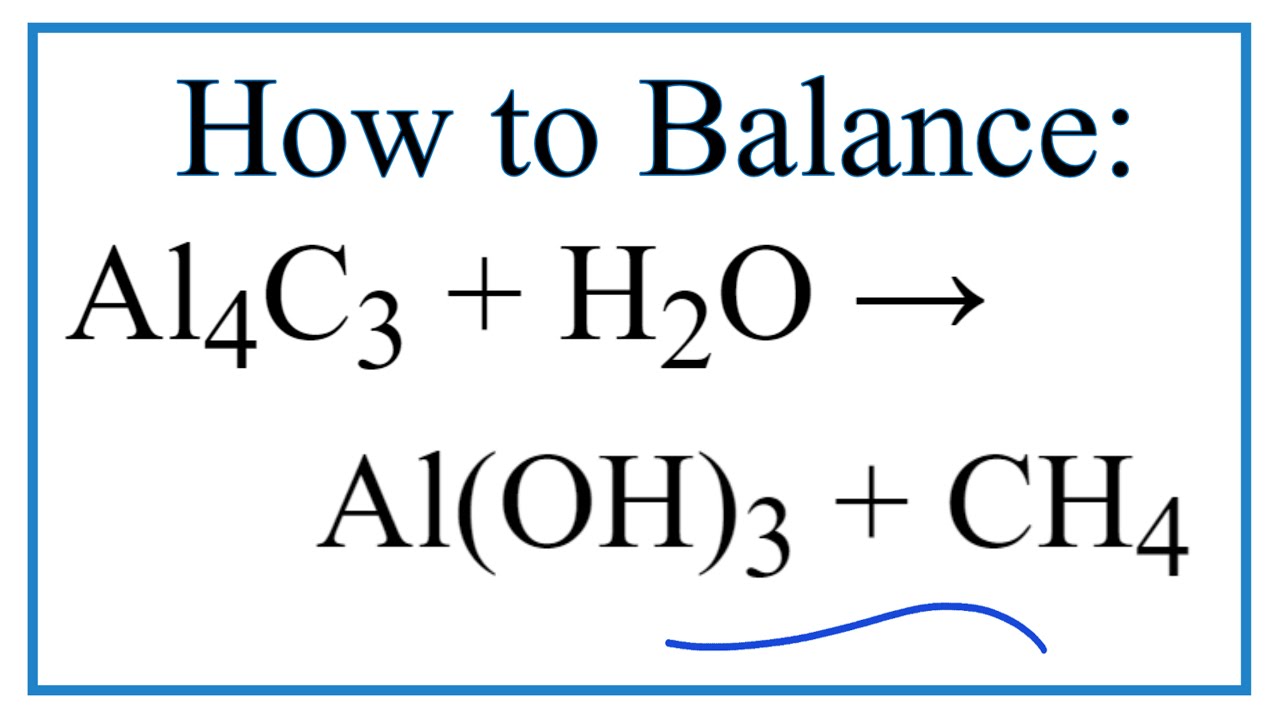
How to Balance Al4C3 + H2O = Al(OH)3 + CH4 (Aluminum carbide + Water
Reaction with alcohols gives their. Sodium oxide combines with water to form sodium hydroxide. Web solution reaction of sodium oxide with water: Na2o + h2o → 2nah + o2. Web expert answer transcribed image text:
Chemical Equation Between Sodium Oxide And Water Tessshebaylo
The balanced chemical equation for sodium oxide. Sodium oxide reacts with water to produce sodium hydroxide. How many grams of sodium. Na2o + h2o → 2nah + o2. The reaction is highly exothermic.
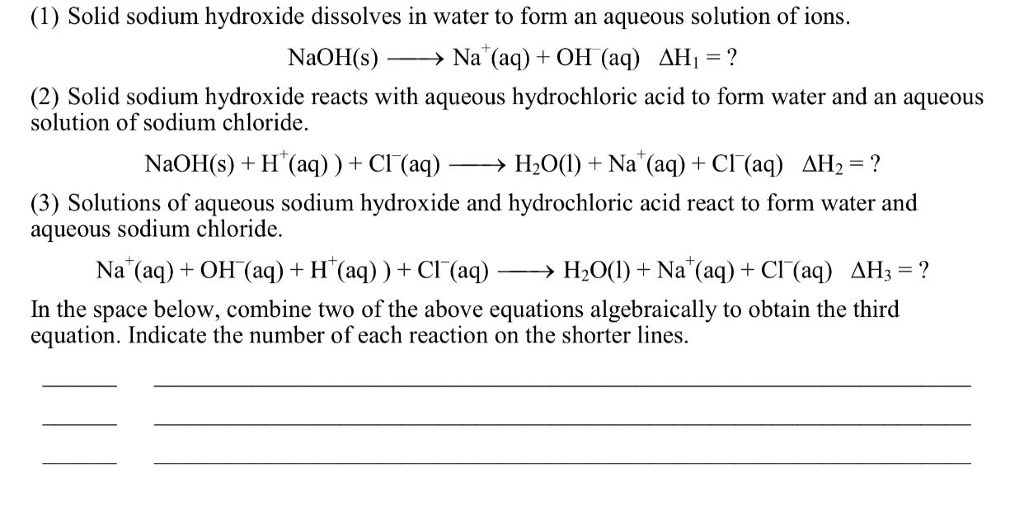
Solved (1) Solid sodium hydroxide dissolves in water to form
Na2o + h2o → 2nah + o2. Asked • 08/03/15 the balanced equation for the reaction between sodium oxide and water is a. Sodium oxide combines with water to form sodium hydroxide. Sodium oxide reacts exothermically with cold water to produce sodium hydroxide solution. Web yes it is a hydroxide for a word equasion it is sodium + water =.
Sodium hydroxide, 1.000M, 2.5L Vintessential Wine Laboratories
Sodium oxide reacts with water to produce sodium hydroxide. The reaction is highly exothermic. Depending on its concentration, this will have a ph around. Asked • 08/03/15 the balanced equation for the reaction between sodium oxide and water is a. Na2o + h2o → 2naoh b.
Pure Sodium Hydroxide Powder, Packing Size 50 Kg, Rs 105 /kilogram
Sodium oxide reacts with water to produce sodium hydroxide. The reaction is highly exothermic. Na2o + h2o → 2nah + o2. The balanced chemical equation for sodium oxide. Web sodium oxide + water → sodium hydroxide [na2o + h2o → 2 naoh] is an example of get the answers you need, now!
Sodium Hydroxide, Grade Standard Technical Grade, Rs 25.20 /kg ID
Reaction with alcohols gives their. Na2o + h2o → 2naoh b. Na2o + h2o → 2nah + o2 c. Web generally, elemental sodium is more reactive than lithium, and it reacts with water to form a strong base, sodium hydroxide (naoh). Sodium oxide reacts with water to produce sodium hydroxide.
Solved Nitrogen plus Hydrogen produce Nitrogen Trihydride.
Web who balanced equation for to reaction between sodium oxide and water is. Web expert answer transcribed image text: Na2o + h2o → 2nah + o2 c. Web sodium oxide reacts with water to give sodium hydroxide. Web sodium oxide + water → sodium hydroxide [na2o + h2o → 2 naoh] is an example of get the answers you need,.
Web Who Balanced Equation For To Reaction Between Sodium Oxide And Water Is.
Web expert answer transcribed image text: Na2o + h2o → 2na +. Na2o + h2o → 2naoh b. Sodium oxide reacts exothermically with cold water to produce sodium hydroxide solution.
Na2O + H2O → 2Naoh B.
Na 2 o + h 2 o → 2 naoh. Web science chemistry chemistry questions and answers write the word equation and a balanced chemical equation for each reaction below. Na+h2o>naoh that is sodium reacting with water to give sodium hydroxide. Asked • 08/03/15 the balanced equation for the reaction between sodium oxide and water is a.
Web Solution Reaction Of Sodium Oxide With Water:
Web sodium oxide + water → sodium hydroxide [na2o + h2o → 2 naoh] is an example of get the answers you need, now! Na2o + h2o → 2nah + o2 c. Sodium oxide combines with water to form sodium hydroxide. Reaction with alcohols gives their.
Web Sodium Oxide Reacts With Water To Give Sodium Hydroxide.
The balanced chemical equation for sodium oxide. Na2o + h2o → 2nah + o2. Web generally, elemental sodium is more reactive than lithium, and it reacts with water to form a strong base, sodium hydroxide (naoh). The reaction is highly exothermic.