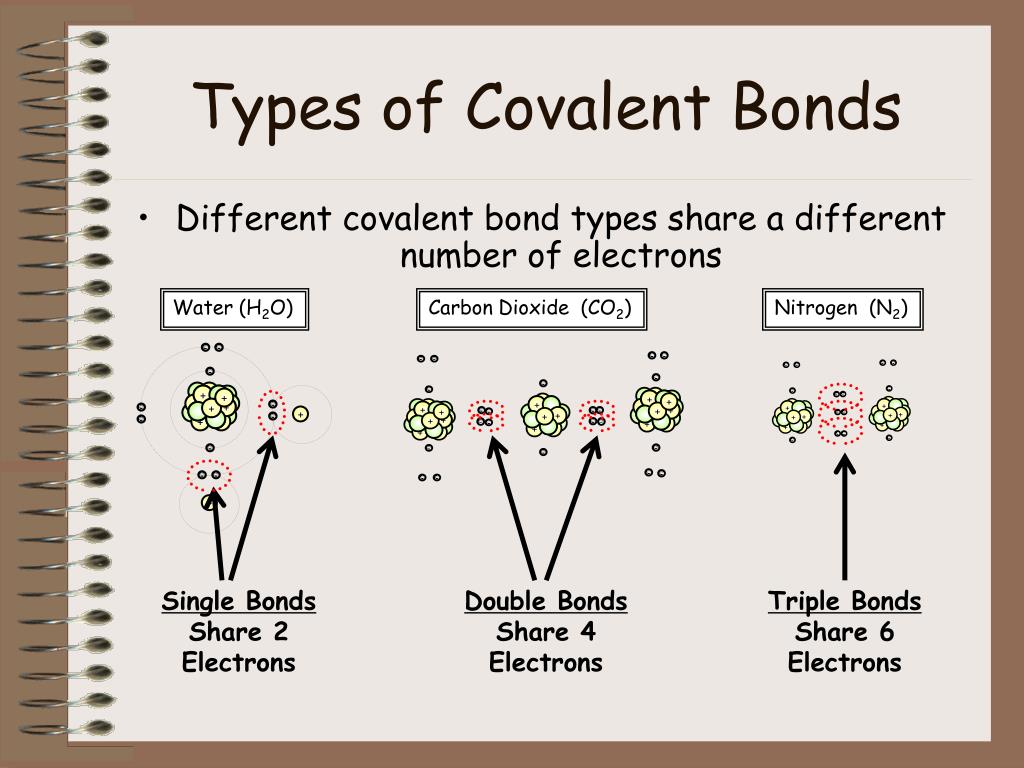
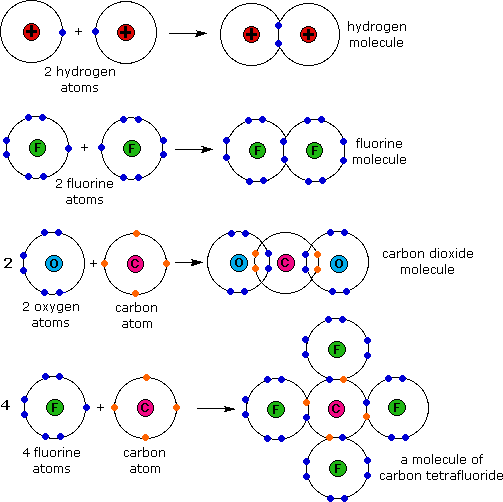
What Types Of Atoms Form Covalent Bonds
What Types Of Atoms Form Covalent Bonds - How does a covalent bond form? Web the two atoms can also share two pairs of electrons (a double bond) or three pairs of electrons (triple bond): Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. Web formation of covalent bonds. Each atom contributes one electron to the shared pair, helping both atoms achieve an octet in their valence shell. Ionic bonds require at least one electron donor and one electron. Web covalent bonds involve the sharing of electron pairs between atoms. In covalent compounds, atoms form covalent bonds that consist of electron pairs shared between two adjacent atomic nuclei. Web a covalent bond is formed when electrons from both participating atoms are shared equally. Each h atom starts with a single electron in its valence shell:
Molecules of identical atoms, such as h 2 and buckminsterfullerene (c 60 ), are also held together by covalent bonds. Web a covalent bond is formed when electrons from both participating atoms are shared equally. In lewis theory, a pair of electrons, known as a bonding pair, is shared between two atoms to form a covalent bond. The differences between ionic and covalent bonds are explained by the use of scientific models and examples from nature. Web double bonds triple bond. A triple bond is formed when three pairs of electrons are shared between the two participating atoms. What is a covalent bond? Each h atom starts with a single electron in its valence shell: How does a covalent bond form? A type of chemical bond where two atoms are connected to each other by the sharing of two or more electrons.
Web nonmetal atoms frequently form covalent bonds with other nonmetal atoms. Starting on the far right, we have two separate hydrogen atoms with a particular potential energy, indicated. This type of covalent bond is. Web double bonds triple bond. Various methods of showing a covalent bond. Ionic bonds require at least one electron donor and one electron. The pair of electrons involved in this type of bonding is known as a shared pair or bonding pair. A triple bond is formed when three pairs of electrons are shared between the two participating atoms. Illustrates why this bond is formed. In organic chemistry, when a molecule with a planar ring obeys hückel's rule, where the number of π electrons fit the formula 4 n + 2.
Covalent Bonds Biology for NonMajors I
What is a covalent bond? Each atom contributes one electron to the shared pair, helping both atoms achieve an octet in their valence shell. The differences between ionic and covalent bonds are explained by the use of scientific models and examples from nature. How does a covalent bond form? When atoms of different elements share electrons through covalent bonding, the.
Covalent Bond Biology Dictionary
A type of chemical bond where two atoms are connected to each other by the sharing of two or more electrons. Web molecular shape isomerism in organic compounds there are many types of chemical bonds and forces that bind molecules together. What types of atoms tend to form the following types of. Ionic bonds require at least one electron donor.
PPT Notes 53 Covalent Bonds PowerPoint Presentation, free download
Two different atoms can also share electrons and form covalent bonds. Any object (such as a magnet, polar molecule or antenna), that is oppositely charged at two points (or poles). Do a covalent bond should necessarily have a difference in their electronegativities. Molecular bonds are another name for covalent bonds. Ionic bonds require at least one electron donor and one.
Polar Covalent Bonds
The electrons involved are in the outer shells of the atoms. The simplest covalent bond exists in the diatomic hydrogen molecule. Web nonmetal atoms frequently form covalent bonds with other nonmetal atoms. A type of chemical bond where two atoms are connected to each other by the sharing of two or more electrons. In lewis theory, a pair of electrons,.
covalent bond Definition, Properties, Examples, & Facts Britannica
A type of chemical bond where two atoms are connected to each other by the sharing of two or more electrons. This type of covalent bond is. A covalent bond is the force of attraction that holds together two nonmetal atoms that share a pair of electrons. It is fundamental to know the bonding characteristics of atoms. Living things are.
Covalent Enseñanza de química, Enlace químico, Enlace covalente
Positively charged and negatively charged parts of covalent molecules attract c. Each type of bond is described below. Hydrogen bonds and london dispersion forces. It is fundamental to know the bonding characteristics of atoms. The electrons involved are in the outer shells of the atoms.
PPT Covalent Bonds PowerPoint Presentation, free download ID6647183
Single covalent bonds between different atoms. What is a covalent bond? For example, the hydrogen molecule, h 2, contains a covalent bond between its two hydrogen atoms. In lewis theory, a pair of electrons, known as a bonding pair, is shared between two atoms to form a covalent bond. Positively charged and negatively charged parts of covalent molecules attract c.
Covalent Bonding (Biology) — Definition & Role Expii
A covalent bond is the force of attraction that holds together two nonmetal atoms that share a pair of electrons. Various methods of showing a covalent bond. Web introduction only when two atoms of the same element form a covalent bond are the shared electrons actually shared equally between the atoms. Starting on the far right, we have two separate.
EduMission Chemistry Form 4 Chapter 5 Covalent Bond
Web introduction only when two atoms of the same element form a covalent bond are the shared electrons actually shared equally between the atoms. Web nonmetal atoms frequently form covalent bonds with other nonmetal atoms. Each atom contributes one electron to the shared pair, helping both atoms achieve an octet in their valence shell. This type of covalent bond exists.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Molecular bonds are another name for covalent bonds. How does a covalent bond form? Web formation of covalent bonds. Each atom contributes one electron to the shared pair, helping both atoms achieve an octet in their valence shell. Two different atoms can also share electrons and form covalent bonds.
Illustrates Why This Bond Is Formed.
Web nonmetal atoms frequently form covalent bonds with other nonmetal atoms. Each atom contributes one electron to the shared pair, helping both atoms achieve an octet in their valence shell. Living things are made up of atoms, but in most cases, those atoms aren’t just floating around individually. What is a covalent bond?
Definition, Functions, Types, And Faqs Jul 7, 2022 Covalent Bond Electronic Configuration Has Been A Very Important Topic In Chemistry Over The Years.
The electrons involved are in the outer shells of the atoms. Web a covalent bond is formed when electrons from both participating atoms are shared equally. Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. Web covalent bonds form between atoms with relatively high electron affinity and they form individual, separate molecules (figure below).
How Does That Work In.
Covalent bonds form between atoms of nonmetallic elements. Web when electrons are shared between two atoms, they form a covalent bond. Web formation of covalent bonds. The pair of electrons involved in this type of bonding is known as a shared pair or bonding pair.
Halogens Also Exist As Diatomic Gases By Forming Covalent Bonds, Such As Chlorine.
A covalent bond forms when_______________. Web double bonds triple bond. In ionic bonding, atoms transfer electrons to each other. Figure 7.4 illustrates why this bond is formed.