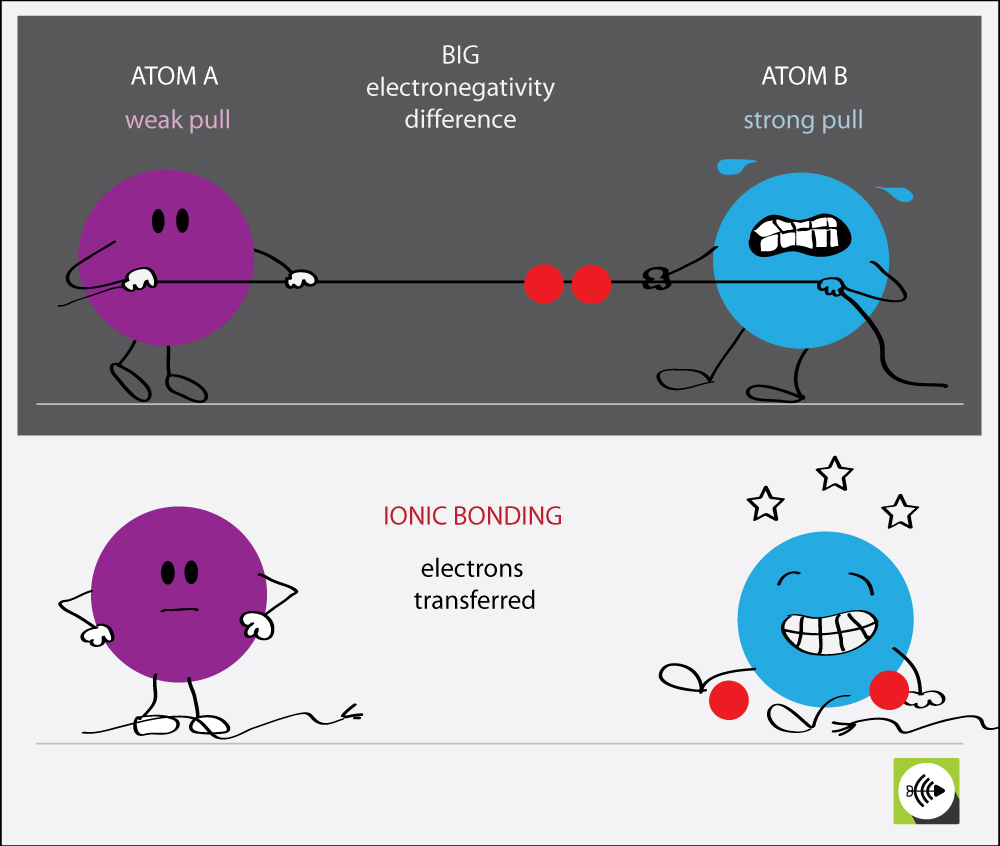
What Types Of Atoms Tend To Form Ionic Bonds
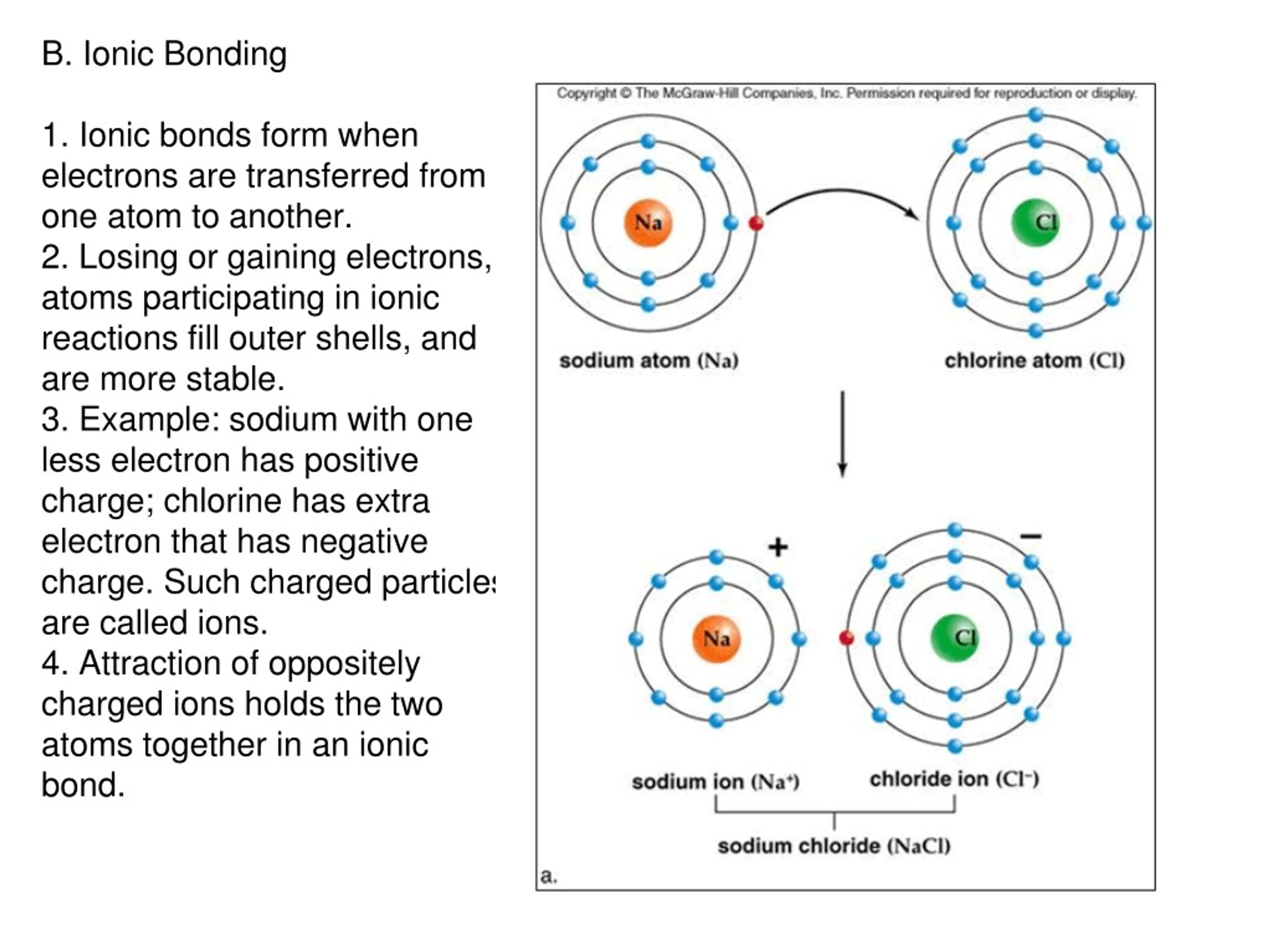
What Types Of Atoms Tend To Form Ionic Bonds - A cation (a positive ion) forms when a neutral atom loses one or more electrons. Electrical conductivity how are ionic bonds formed? Web in order to form neutral compounds, the total charges must be balanced. An atom of sodium will lose an electron and form a positive ion. Ionic bonds are formed by the attraction. The resulting sodium ion has the same electron configuration as. What types of atoms tend to form ionic bonding? Web as you have learned in chapter 2, ions are atoms or molecules bearing an electrical charge. One atom acts as an electron donor, and the other as an electron. Web an ionic bond is a bond between two oppositely charged chemical species which can be singular atoms or a group of atoms.
The resulting sodium ion has the same electron configuration as. Ionic bonds are formed by the attraction. A molecule or compound is made when two or more atoms. An atom of sodium will lose an electron and form a positive ion. Comparison of ionic and covalent bonds. Ionic bond, covalent and metallic bond together. Of the two charged species, one is positively. A cation (a positive ion) forms when a neutral atom loses one or more electrons. Web jan 29, 2023. An atom of chlorine will gain.
A cation (a positive ion) forms when a neutral atom loses one or more electrons. A molecule or compound is made when two or more atoms. Ionic bond, covalent and metallic bond together. Web in forming an ionic bond, the sodium atom, which is electropositive, loses its valence electron to chlorine. Ionic bonding is the complete transfer of valence electron (s) between atoms. An atom of chlorine will gain. Web as you have learned in chapter 2, ions are atoms or molecules bearing an electrical charge. Web ionic compounds generally form from metals and nonmetals. Of the two charged species, one is positively. Web jan 29, 2023.
Difference Between Ionic Covalent and Metallic Bonds Definition
Ionic bonds are formed by the attraction. Ionic bonding is the complete transfer of valence electron (s) between atoms and is a type of chemical. Web jan 29, 2023. An atom of chlorine will gain. Web the 2 main types of bonds that link atoms together to form compounds are covalent and electrovalent/ionic.
Chemical Bonds
Web the 2 main types of bonds that link atoms together to form compounds are covalent and electrovalent/ionic. Ionic bond, covalent and metallic bond together. Comparison of ionic and covalent bonds. One atom acts as an electron donor, and the other as an electron. An atom of sodium will lose an electron and form a positive ion.
Ionic Bond Definition, Types, Properties & Examples
An atom of chlorine will gain. Compounds that do not contain ions, but instead consist of atoms bonded tightly together in. Ionic bond, covalent and metallic bond together. A molecule or compound is made when two or more atoms. Web an ionic bond is a bond between two oppositely charged chemical species which can be singular atoms or a group.
Chemistry form 4 ionic bond
Web as you have learned in chapter 2, ions are atoms or molecules bearing an electrical charge. Web in forming an ionic bond, the sodium atom, which is electropositive, loses its valence electron to chlorine. Ionic bonds are formed by the attraction. What types of atoms tend to form the following types of bonding? What types of atoms tend to.
Electronegativity Bond Scale Surfguppy Chemistry made easy for
Web as you have learned in chapter 2, ions are atoms or molecules bearing an electrical charge. Web in forming an ionic bond, the sodium atom, which is electropositive, loses its valence electron to chlorine. A molecule or compound is made when two or more atoms. What types of atoms tend to form ionic bonding? The resulting sodium ion has.
Ionic Bonds BOOKSTRONAUTS
Web what are three types of bonds that can form between atoms of 2 or more elements to form compounds? Web as you have learned in chapter 2, ions are atoms or molecules bearing an electrical charge. Ionic bonds are formed by the attraction. An atom of sodium will lose an electron and form a positive ion. A cation (a.
PPT 2.1 Chemical Elements PowerPoint Presentation, free download ID
Web ionic and covalent bonds. An atom of chlorine will gain. Ionic bonding is the complete transfer of valence electron (s) between atoms. Of the two charged species, one is positively. One atom acts as an electron donor, and the other as an electron.
Ionic Bond Definition, Types, Properties & Examples
Compounds that do not contain ions, but instead consist of atoms bonded tightly together in. Web what are three types of bonds that can form between atoms of 2 or more elements to form compounds? Ionic bonding is the complete transfer of valence electron (s) between atoms and is a type of chemical. Ionic bonds are formed by the attraction..
chemistry Can carbon and titanium form an ionic bond
Web what are three types of bonds that can form between atoms of 2 or more elements to form compounds? Ionic bonds are formed by the attraction. Electrical conductivity how are ionic bonds formed? A molecule or compound is made when two or more atoms. Web as you have learned in chapter 2, ions are atoms or molecules bearing an.
Is SiO2 Ionic or Covalent? Techiescientist
An atom of sodium will lose an electron and form a positive ion. An atom of chlorine will gain. Of the two charged species, one is positively. Web ionic compounds generally form from metals and nonmetals. Ionic bonding is the complete transfer of valence electron (s) between atoms and is a type of chemical.
Web Ionic Compounds Generally Form From Metals And Nonmetals.
It is a type of chemical bond that generates two. Web an ionic bond is a bond between two oppositely charged chemical species which can be singular atoms or a group of atoms. The octet rule is the concept that atoms tend to have eight electrons in their valence electron shell. An atom of chlorine will gain.
Boiling And Melting Point 4.
Web in order to form neutral compounds, the total charges must be balanced. One atom acts as an electron donor, and the other as an electron. Compounds that do not contain ions, but instead consist of atoms bonded tightly together in. Ionic bonds are formed by the attraction.
The Resulting Sodium Ion Has The Same Electron Configuration As.
What types of atoms tend to form ionic bonding? Ionic bonds are formed by the attraction. Comparison of ionic and covalent bonds. The octet rule is the concept that atoms tend to have eight electrons in their valence electron shell.
Web What Are Three Types Of Bonds That Can Form Between Atoms Of 2 Or More Elements To Form Compounds?
Ionic bonding is the complete transfer of valence electron (s) between atoms and is a type of chemical. Ionic bond, covalent and metallic bond together. Web jan 29, 2023. Electrical conductivity how are ionic bonds formed?
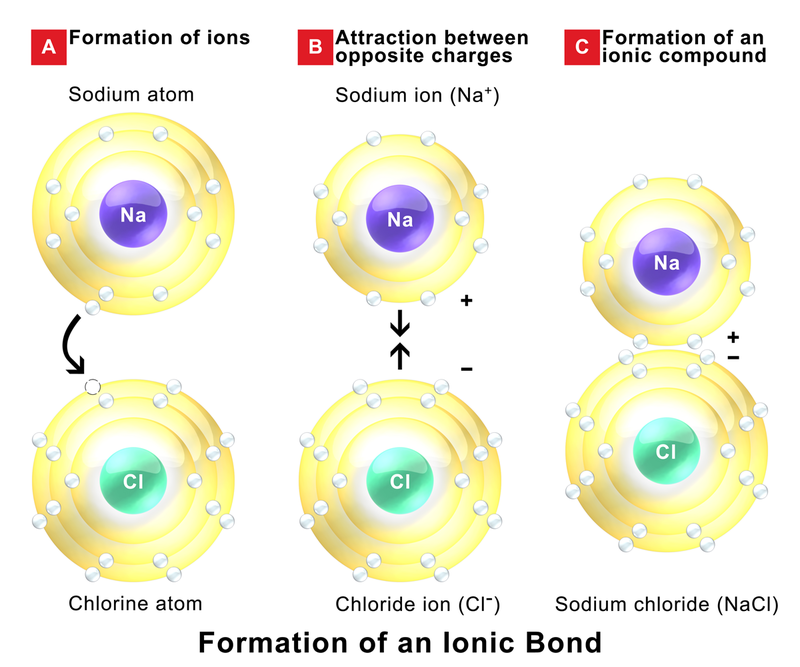
.PNG)