When Metals Form Ions They Tend To Do So By
When Metals Form Ions They Tend To Do So By - Transition metals often form more than one. Web compounds that contain ions are called ionic compounds. Groups 1 and 2 are called the alkali. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the. Web as you may notice, they can form ions by either losing or gaining electron in 4s orbital. Ionic compounds generally form from metals and nonmetals. Web so my question is: Web metals form ions by losing electrons. Losing electrons and forming positive ionsexplanation:metals form ions by losing electrons. Web compounds that contain ions are called ionic compounds.
Web metals form ions by losing electrons. Transition metals often form more than one. When metals form ions they tend to do so by1. Web compounds that contain ions are called ionic compounds. So rather than accept more electrons, it is much more. Web metal atoms lose electrons from their outer shell when they form ions: Thus metals are electropositive elements. Web metals tend to form: Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the. Compounds that do not contain ions, but instead consist of.
Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the. A cations b anions c cations and anions d do not form ions easy open in app solution verified by toppr correct option is a) metals lose electrons in. Web metal atoms lose electrons from their outer shell when they form ions: Metals tend to form cations, while nonmetals tend to form anions. Thus metals are electropositive elements. Losing electrons and forming positive ions2. Web atoms gain or lose electrons to form ions with particularly stable electron configurations. Losing electrons and forming positive ionsexplanation:metals form ions by losing electrons. This is actually one of the chemical properties of metals and nonmetals: Is there a simple way to tell which elements have multiple cations, or is it pure memorization?
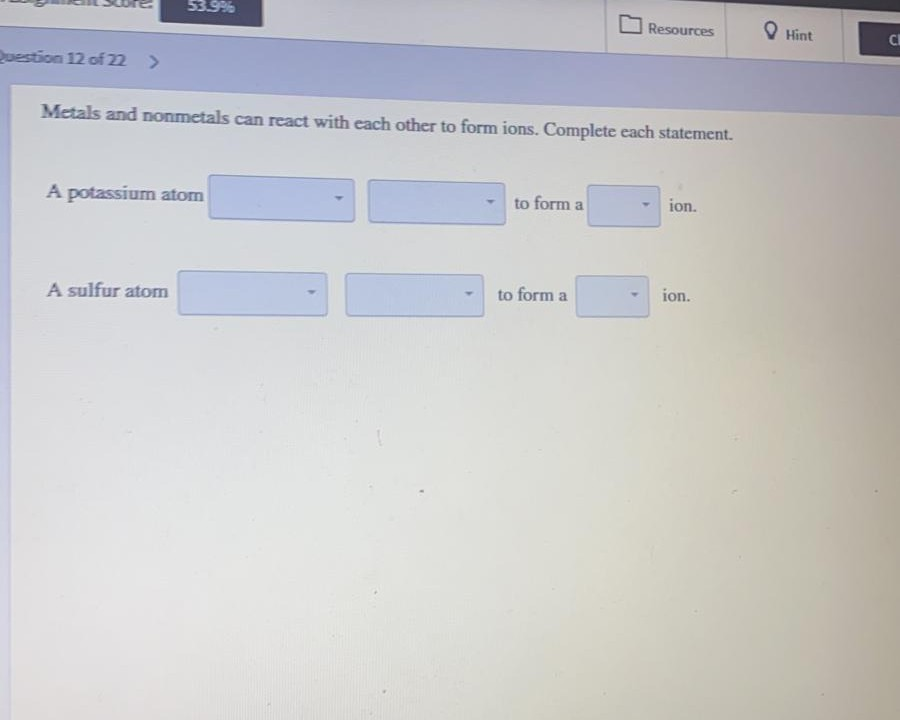
Solved 53.9 Resources Hint СІ Question 12 of 22 > Metals
Web so my question is: The charges of cations formed by the representative metals may be determined readily. Transition metals often form more than one. Web compounds that contain ions are called ionic compounds. Web as you may notice, they can form ions by either losing or gaining electron in 4s orbital.
Colours of Transition Metal Ions in Aqueous Solution Compound Interest
Thus metals are electropositive elements. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the. Metals tend to form cations, while nonmetals tend to form anions. Ionic compounds generally form from metals and nonmetals. Web with the exception of hydrogen, all elements.
Student Resources CHEMISTRY BATZ
Web metals tend to form positive ions because their electron structure causes them to do so. Losing electrons and forming positive ions2. Ionic compounds generally form from metals and nonmetals. When metals form ions they tend to do so by1. Web atoms gain or lose electrons to form ions with particularly stable electron configurations.
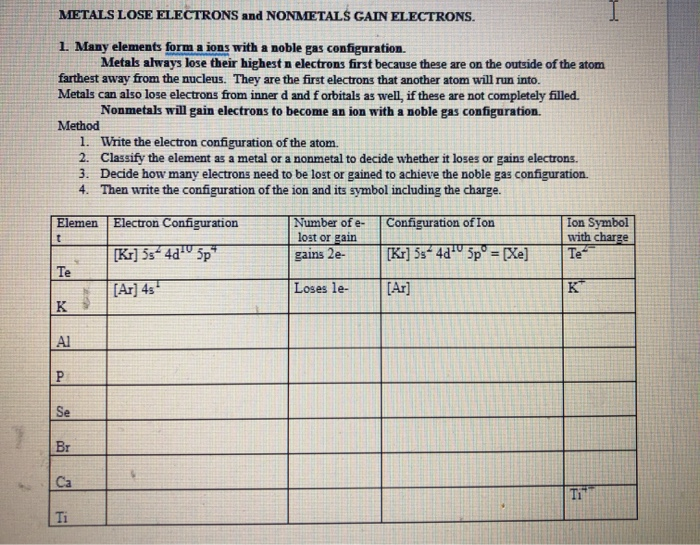
Solved METALS LOSE ELECTRONS and NONMETALS GAIN ELECTRONS.
Web compounds that contain ions are called ionic compounds. Metal elements form positively charged ions called cations because they are located on the left side of the periodic table. Web answer (1 of 4): Web metals tend to form: Ionic compounds generally form from metals and nonmetals.
table of elements chart
Metals tend to form cations, while nonmetals tend to form anions. Ionic compounds generally form from metals and nonmetals. Transition metals often form more than one. Web metal atoms lose electrons from their outer shell when they form ions: Web so my question is:
Pin em Science, bitch!
Web so my question is: Web with the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. When metals form ions they tend to do so by1. Web answer (1 of 4): The charges of cations formed by the representative metals may be determined readily.
2.6 Ionic Compounds and Formulas Chemistry LibreTexts
Thus metals are electropositive elements. When metals form ions they tend to do so by1. Web metals tend to form: We need to talk briefly about what this means, so put on your thinking. Metals tend to form cations, while nonmetals tend to form anions.
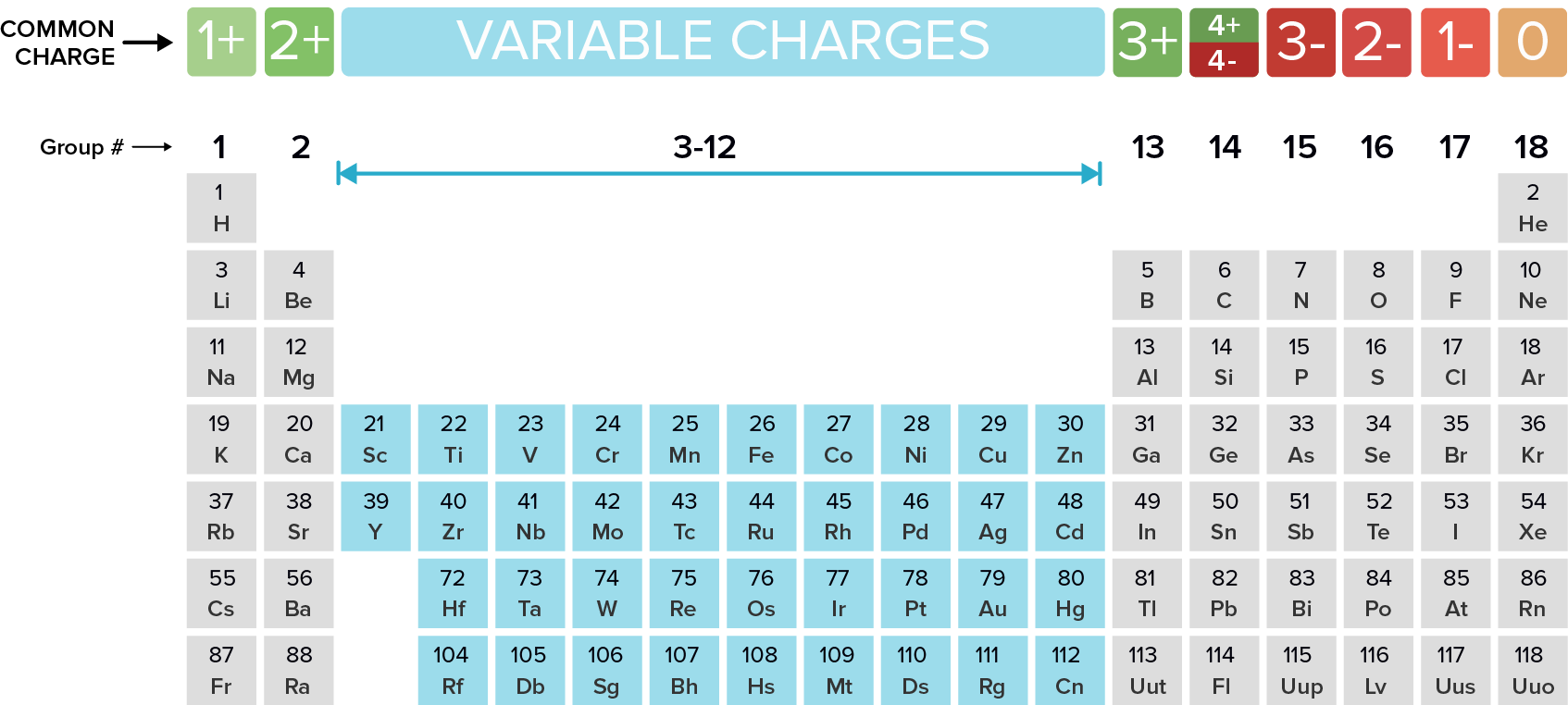
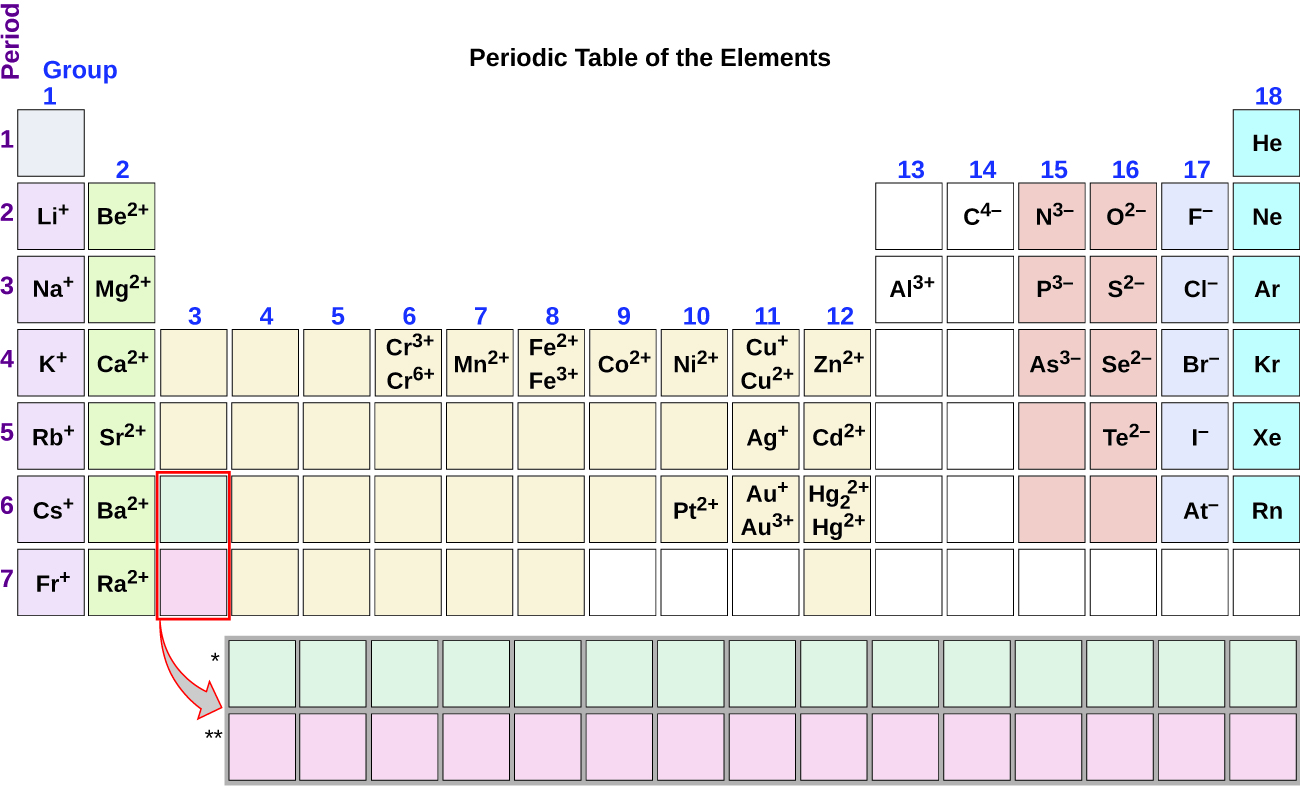
Ion Charge from Periodic Table NemoQuiz
Web compounds that contain ions are called ionic compounds. Web compounds that contain ions are called ionic compounds. Web metals tend to form positive ions because their electron structure causes them to do so. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges.
CH103 CHAPTER 4 Ions and Ionic Compounds Chemistry
Web metals form ions by losing electrons. Web compounds that contain ions are called ionic compounds. Ionic compounds generally form from metals and nonmetals. Web in general, when metals form an ion, it is easier for them to lose electrons to attain a stable noble gas electronic configuration, as most metals have electrons. Web metals tend to form:
Use data above answer questions 2,3,4 EXPERIMENT Activity of Metals
Groups 1 and 2 are called the alkali. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the. Losing electrons and forming positive ionsexplanation:metals form ions by losing electrons. Web metal atoms lose electrons from their outer shell when they form ions:.
Web Atoms Gain Or Lose Electrons To Form Ions With Particularly Stable Electron Configurations.
Ionic compounds generally form from metals and nonmetals. They do it because they have few electrons in their outermost shells. We need to talk briefly about what this means, so put on your thinking. Web with the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals.
Web Metals Form Ions By Losing Electrons.
A cations b anions c cations and anions d do not form ions easy open in app solution verified by toppr correct option is a) metals lose electrons in. Losing electrons and forming positive ions2. Metals tend to form cations, while nonmetals tend to form anions. Losing electrons and forming positive ionsexplanation:metals form ions by losing electrons.
The Ions Are Positive, Because They Have More Protons Than Electrons The Ions Formed Have Full Outer.
Web compounds that contain ions are called ionic compounds. The charges of cations formed by the representative metals may be determined readily. So rather than accept more electrons, it is much more. Web answer (1 of 4):
Web Metals Tend To Form Positive Ions Because Their Electron Structure Causes Them To Do So.
Transition metals often form more than one. Web so my question is: Web they do it because they have few electrons in their outermost answer:1. Web as you may notice, they can form ions by either losing or gaining electron in 4s orbital.