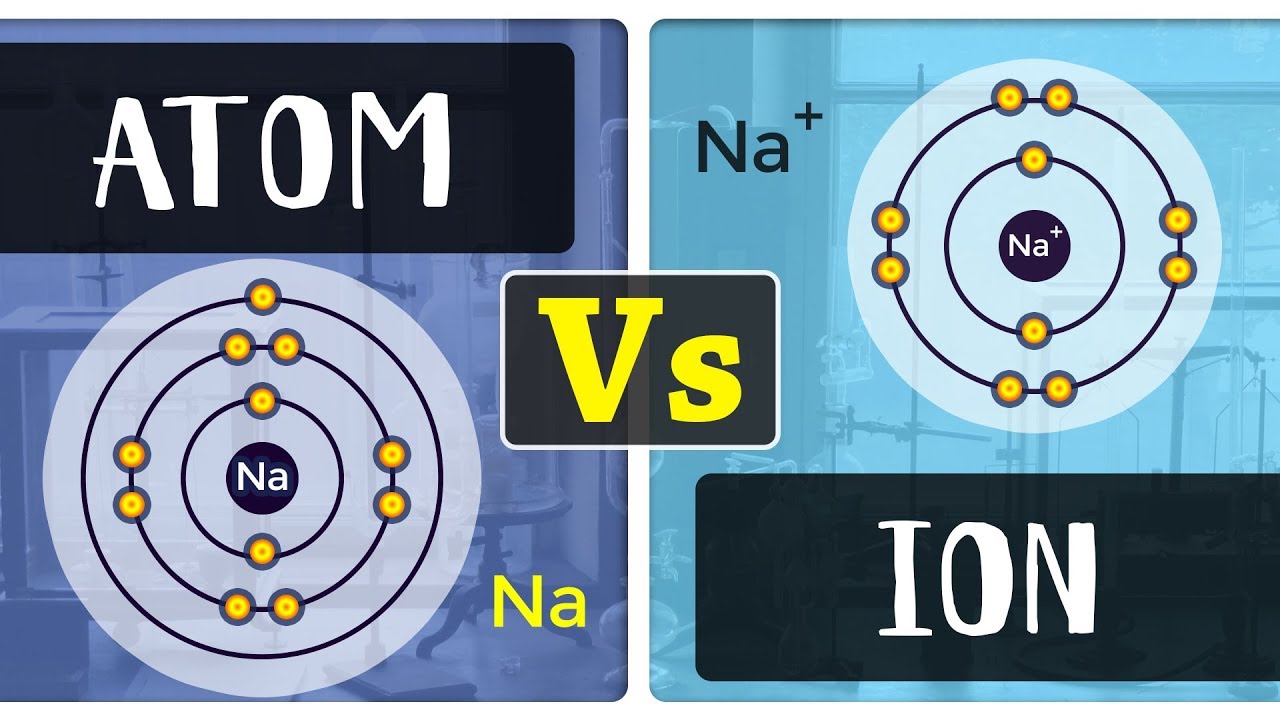
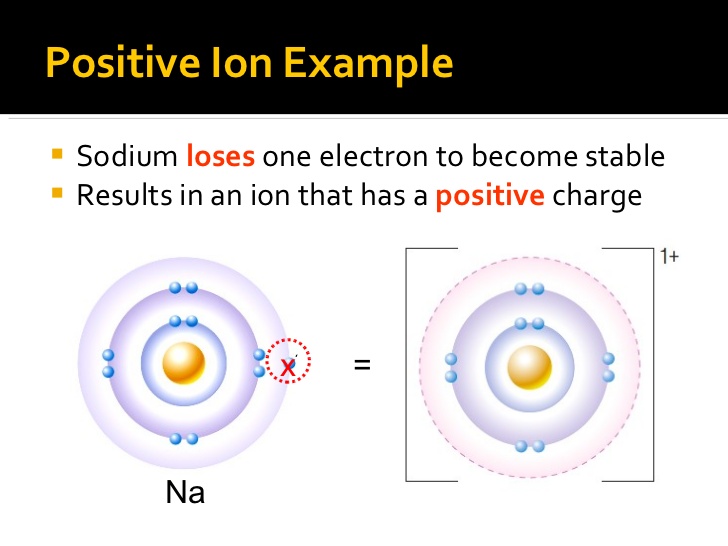
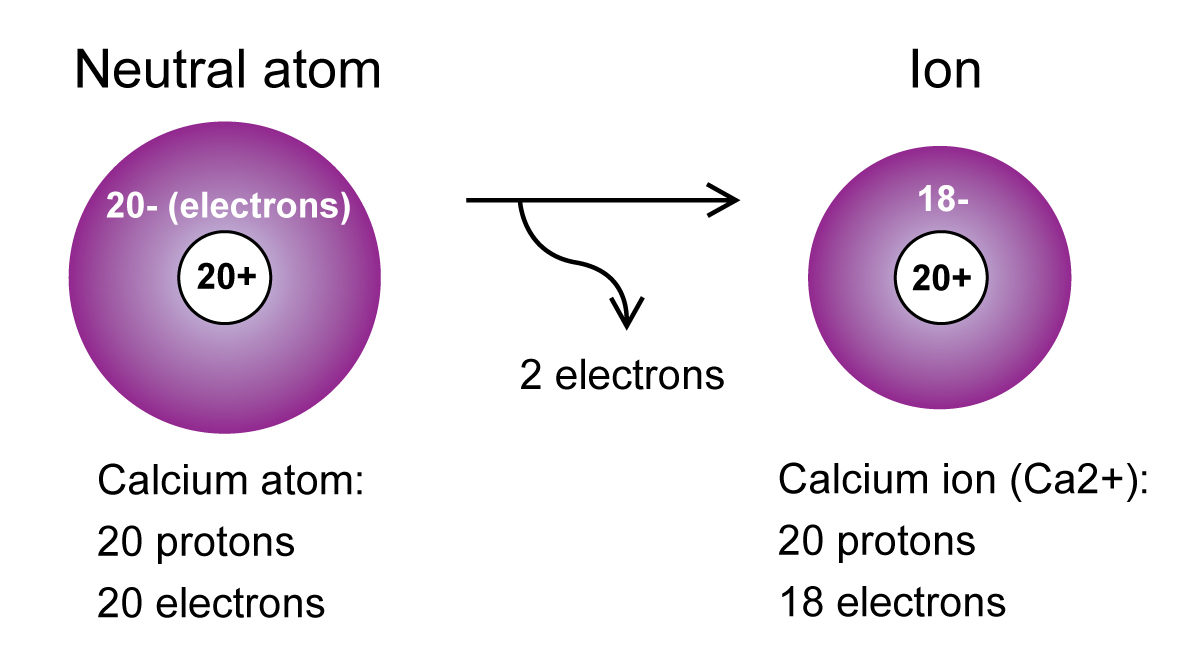
Which Ion Is This Atom Most Likely To Form
Which Ion Is This Atom Most Likely To Form - Group 1 and group 7 atoms. An ion is an atom with a. Web which atom is most likely to accept electrons to form an ionic bond? Web which ion is this atom most likely to form? Correct answer is option 4; You'll get a detailed solution from a subject matter expert that helps. Web part of combined science bonding, structure and the properties of matter revise video test 1 2 3 4 forming ions an ion is an atom or group of atoms with a positive or negative. For every 15 x atoms present in this compound, how many y atoms are there? You'll get a detailed solution. Web which ion is this atom most likely to form?
Web which atom is most likely to accept electrons to form an ionic bond? Web what atoms are most likely to form ions? Sulfur, a nonmetal with 6 valence electrons which two elements will most likely form an ionic bond? What charge (in brackets) is likely on a monatomic silver cation? You'll get a detailed solution. Group 1 and group 7 atoms. An ion is an atom with a. Web up to $3 cash back get the detailed answer: Web types of ions: Which atom is most likely to form a 2+ ion?
Which atom is most likely to accept electrons to form an ionic bond? Which atom is most likely to form a 2+ ion? Sulfur, a nonmetal with 6 valence electrons which two elements will most likely form an ionic bond? This is so because sulphur belon. See answer (1) best answer. Web which atom is most likely to form a 3+ ion? Web what atoms are most likely to form ions? Web which ion is this atom most likely to form? (3s) na+ a compound has the formula x3y. Sulfur, a nonmetal with 6 valence electrons which two elements will most likely form an ionic bond?.
table of elements chart
Correct answer is option 4; Web what atoms are most likely to form ions? Which atom is most likely to form a 2+ ion? Web which atom is most likely to accept electrons to form an ionic bond? A potassium ion with a negative 1 charge.
Ion Hydronium Ion American Chemical Society / Ions migrate under the
Sulfur, a nonmetal with 6 valence electrons which two elements will most likely form an ionic bond?. What charge (in brackets) is likely on a monatomic silver cation? A potassium ion with a negative 1 charge. You'll get a detailed solution. Group 1 and group 7 atoms.
Molecular and Ionic Compounds Chemistry I
Lesson summary how are ions formed? Web which ion is this atom most likely to form? Web learn test match created by breanna5225 terms in this set (67) according to this diagram, when lithium and fluorine form an ionic bond, the fluorine is turned into the element. This is so because sulphur belon. Sulfur, a nonmetal with 6 valence electrons.
What happens if an atom is not neutral? Socratic
A 1) zn 2)s k 3) f 4) p 105) ca 5 this problem has been solved! Lesson summary how are ions formed? An atom is composed of protons, neutrons,. This is so because sulphur belon. Web up to $3 cash back get the detailed answer:
Ion Hydronium Ion American Chemical Society / Ions migrate under the
You'll get a detailed solution. Web learn test match created by breanna5225 terms in this set (67) according to this diagram, when lithium and fluorine form an ionic bond, the fluorine is turned into the element. Lesson summary how are ions formed? Sulfur, a nonmetal with 6 valence electrons which two elements will most likely form an ionic bond? Web.
Question 6b789 Socratic
What charge (in brackets) is likely on a monatomic silver cation? Correct answer is option 4; Cations and anions how to determine the charge of an ion? Web which atom is most likely to accept electrons to form an ionic bond? A 1) zn 2)s k 3) f 4) p 105) ca 5 this problem has been solved!
molecular orbital theory How are the hydrogens attached to the
Web which atom is most likely to accept electrons to form an ionic bond? Group 1 and group 7 atoms. You'll get a detailed solution from a subject matter expert that helps. Lesson summary how are ions formed? Web which ion is this atom most likely to form?
Lewis Structures of Ions Mr Pauller YouTube
Web which ion is this atom most likely to form? You'll get a detailed solution from a subject matter expert that helps. Which atom is most likely to accept electrons to form an ionic bond? Lesson summary how are ions formed? Cations and anions how to determine the charge of an ion?
What Does Ion Mean? Slanguide
An atom is composed of protons, neutrons,. An ion is an atom with a. Sulfur, a nonmetal with 6 valence electrons which two elements will most likely form an ionic bond?. Correct answer is option 4; What charge (in brackets) is likely on a monatomic silver cation?
What are difference between atom and ion? Definition, Types and
A mercury ion with a negative 2 charge. Which atom is most likely to accept electrons to form an ionic bond? Sulfur, a nonmetal with 6 valence electrons which two elements will most likely form an ionic bond?. Which atom is most likely to form a 2+ ion? Web which atom is most likely to accept electrons to form an.
Web Types Of Ions:
A potassium ion with a negative 1 charge. Web which atom is most likely to accept electrons to form an ionic bond? An ion is an atom with a. Sulfur, a nonmetal with 6 valence electrons which two elements will most likely form an ionic bond?.
Web Which Ion Is This Atom Most Likely To Form?
(3s) na+ a compound has the formula x3y. What charge (in brackets) is likely on a monatomic silver cation? You'll get a detailed solution from a subject matter expert that helps. Group 1 and group 7 atoms.
Cations And Anions How To Determine The Charge Of An Ion?
You'll get a detailed solution. Web which ion is this atom most likely to form? Web learn test match created by breanna5225 terms in this set (67) according to this diagram, when lithium and fluorine form an ionic bond, the fluorine is turned into the element. Web which atom is most likely to accept electrons to form an ionic bond?
Correct Answer Is Option 4;
Web what atoms are most likely to form ions? Lesson summary how are ions formed? Which atom is most likely to accept electrons to form an ionic bond? See answer (1) best answer.