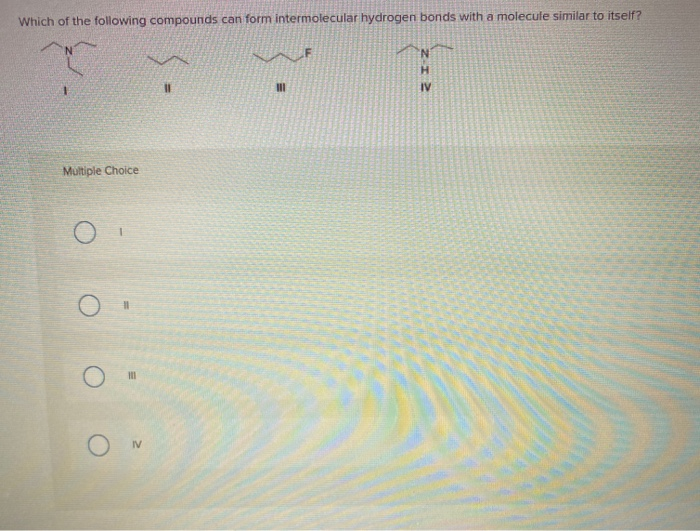
Which Of The Following Compounds Can Form Intermolecular Hydrogen Bonds
Which Of The Following Compounds Can Form Intermolecular Hydrogen Bonds - B) h:se c) nh3 d) h2 e) all of these compounds can form hydrogen bonds. Which of the following compounds. Web which of the following compounds would be expected to form intermolecular hydrogen bonds in the liquid state? Ch3och3 b.ch4 c.hf d.ch3co2h e.br2 f.ch3oh. Web which of the following compounds will form intermolecular hydrogen bonds in the liquid state? Web thus, hydrogen bonds are a very special class of intermolecular attractive forces that arise only in compounds featuring hydrogen atoms bonded to a highly electronegative. Web which of the following compounds can form intermolecular hydrogen bonds? (select all that apply.) hf br2 ch3oh ch4 Web click here👆to get an answer to your question ️ which of the following compounds would have significant intermolecular hydrogen bonding? Web consider the intermolecular forces present in a pure sample of each of the following compounds:
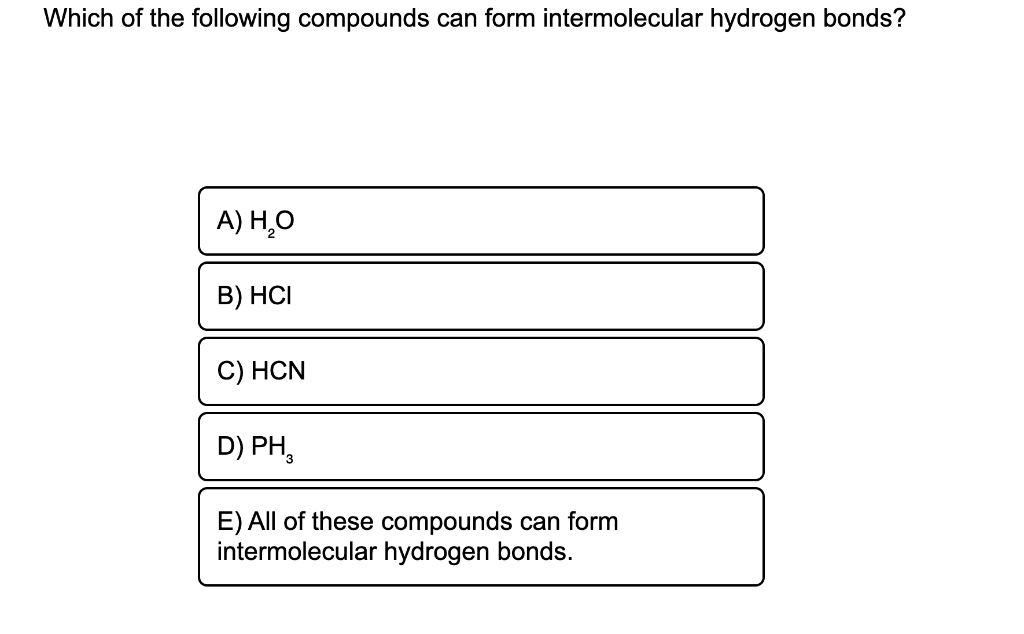
E) all of these can form intermoleculat hydrogen bonds Which of the following compounds. A) h2se b) hf c) hci d) hbr buy chemistry in focus 7th edition isbn: A) h₂se b) hf c) hcl d) hbr e) all of these compounds can form hydrogen bonds. Web which of the following compounds can form intermolecular hydrogen bonds? B) h:se c) nh3 d) h2 e) all of these compounds can form hydrogen bonds. A) h20 b) hci c) hcn d) ph3 e) all of these. E) all of these compounds can form. View the full answer transcribed image text: Web which of the following compounds can form intermolecular hydrogen bonds?
Which of the following compounds. Web which of the following compounds would be expected to form intermolecular hydrogen bonds in the liquid state? (a) ch3och3(dimethyl ether), (b) ch4(c) hf, (d). >> chemical bonding and molecular structure. Web consider the intermolecular forces present in a pure sample of each of the following compounds: (select all that apply.) hf br2 ch3oh ch4 B) h:se c) nh3 d) h2 e) all of these compounds can form hydrogen bonds. Web hydrogen bonding in organic molecules containing nitrogen. Web science chemistry chemistry questions and answers which of the following compounds can form intermolecular hydrogen bonds with a molecule identical to itself (electron. E) all of these compounds can form.
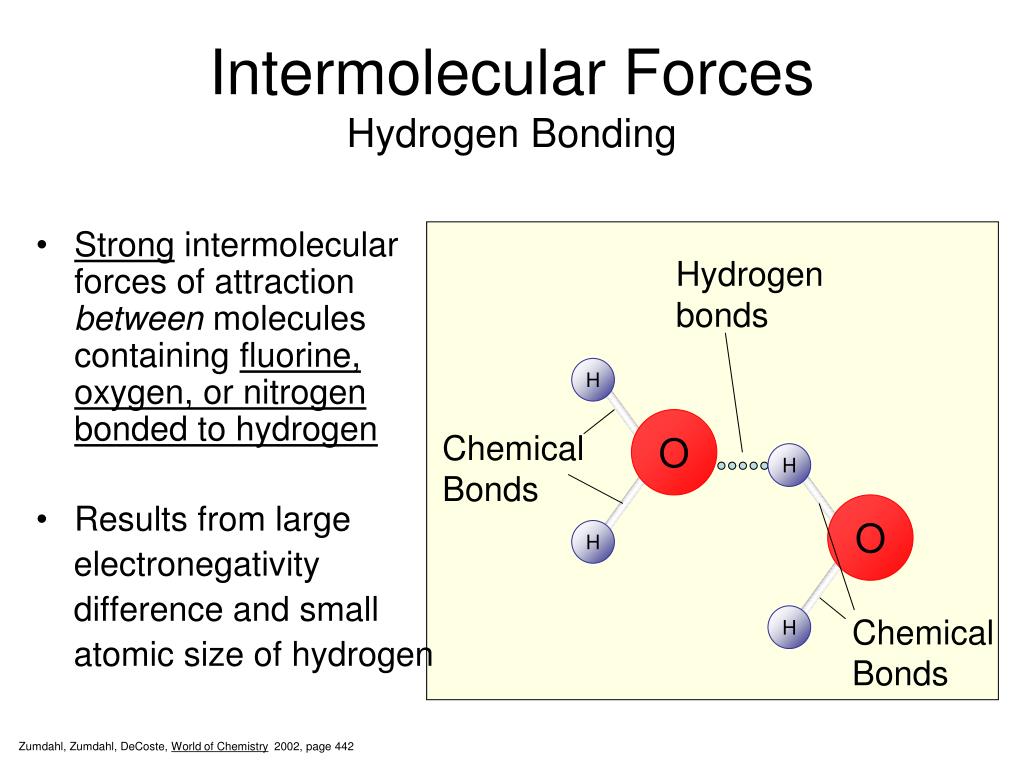
Difference Between Intermolecular and Intramolecular Hydrogen Bonding
Web which of the following compounds can form intermolecular hydrogen bonds? Web which of the following compounds would be expected to form intermolecular hydrogen bonds in the liquid state? Web which of the following compounds can form intermolecular hydrogen bonds? A) h₂se b) hf c) hcl d) hbr e) all of these compounds can form hydrogen bonds. Web 8.40 which.
Solved Which of the following compounds can form
B) h:se c) nh3 d) h2 e) all of these compounds can form hydrogen bonds. A) h20 b) hci c) hcn d) ph3 e) all of these. Web 8.40 which of the following compounds would be expected to form intermolecular hydrogen bonds in the liquid state? E) all of these compounds can form. Ch3och3 b.ch4 c.hf d.ch3co2h e.br2 f.ch3oh.
PPT Intermolecular Forces PowerPoint Presentation ID705859
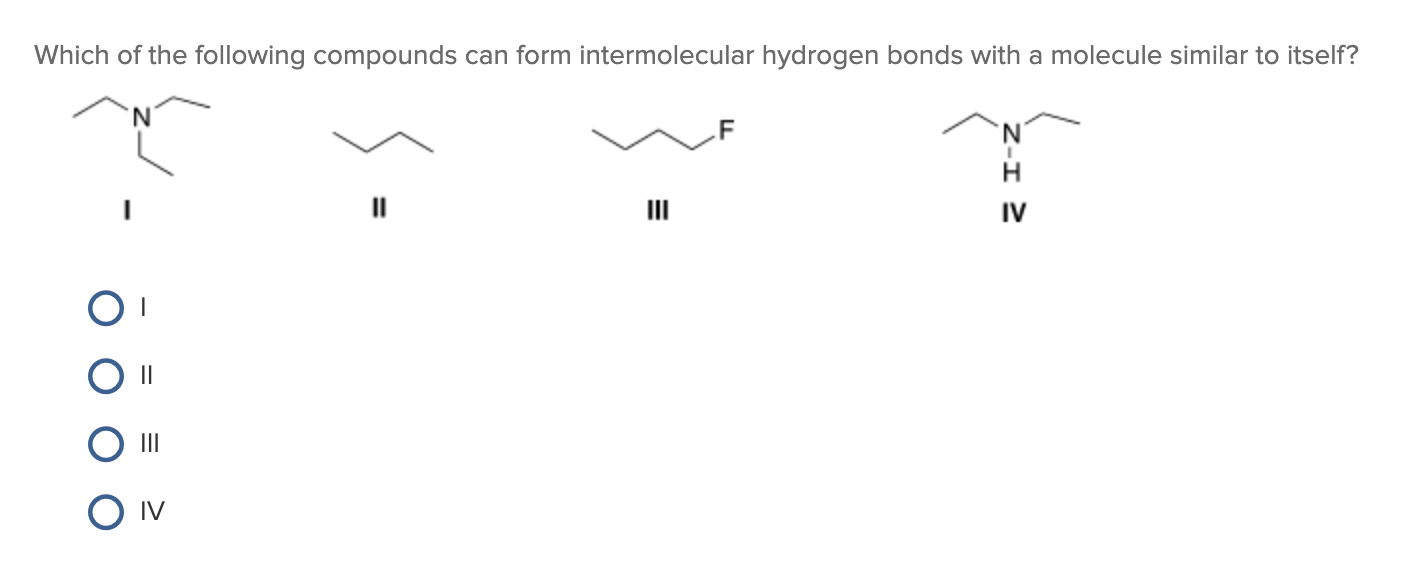
Web science chemistry chemistry questions and answers which of the following compounds can form intermolecular hydrogen bonds with a molecule identical to itself (electron. Web which of the following compounds can form intermolecular hydrogen bonds? (a) ch3och3(dimethyl ether), (b) ch4(c) hf, (d). Web hydrogen bonding in organic molecules containing nitrogen. Web thus, hydrogen bonds are a very special class of.
Diagram Of Water Molecules Hydrogen Bonding
Web hydrogen bonding in organic molecules containing nitrogen. (a) ch3och3(dimethyl ether), (b) ch4(c) hf, (d). Ch3och3 b.ch4 c.hf d.ch3co2h e.br2 f.ch3oh. >> chemical bonding and molecular structure. Which of the following compounds.
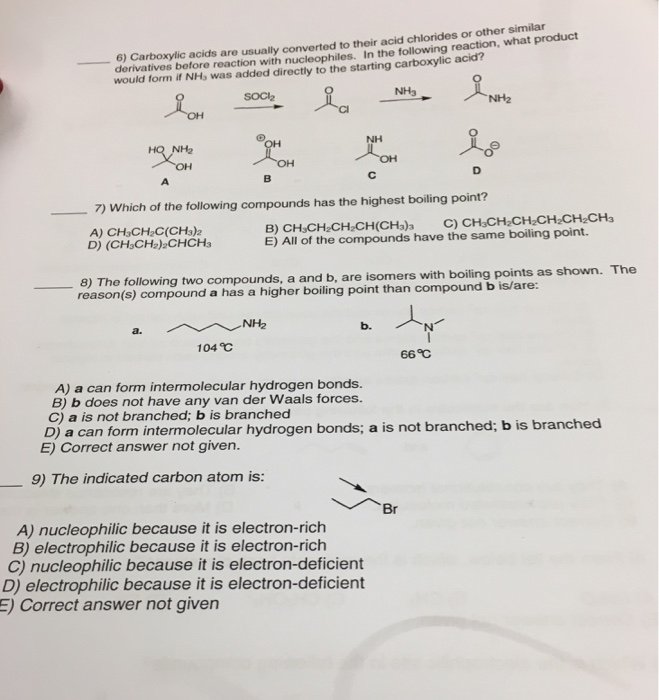
Solved Carboxylic Acids Are Usually Converted To Their Ac...
E) all of these can form intermoleculat hydrogen bonds Which of the following compounds. Web thus, hydrogen bonds are a very special class of intermolecular attractive forces that arise only in compounds featuring hydrogen atoms bonded to a highly electronegative. Web click here👆to get an answer to your question ️ which of the following compounds would have significant intermolecular hydrogen.
Solved 8. Which of the following compounds would be expected
Web 8.40 which of the following compounds would be expected to form intermolecular hydrogen bonds in the liquid state? Web thus, hydrogen bonds are a very special class of intermolecular attractive forces that arise only in compounds featuring hydrogen atoms bonded to a highly electronegative. View the full answer transcribed image text: Web science chemistry chemistry questions and answers which.
Solved Which of the following compounds can form
Web which of the following compounds can form intermolecular hydrogen bonds? Ch3och3 b.ch4 c.hf d.ch3co2h e.br2 f.ch3oh. View the full answer transcribed image text: Web which of the following compounds can form intermolecular hydrogen bonds? E) all of these can form intermoleculat hydrogen bonds
Solved Which Of The Following Compounds Can Form Intermol...
Web thus, hydrogen bonds are a very special class of intermolecular attractive forces that arise only in compounds featuring hydrogen atoms bonded to a highly electronegative. Web click here👆to get an answer to your question ️ which of the following compounds would have significant intermolecular hydrogen bonding? Web which of the following compounds can form intermolecular hydrogen bonds? A) h2se.
Image result for intermolecular forces
Web which of the following compounds would be expected to form intermolecular hydrogen bonds in the liquid state? E) all of these compounds can form. A) h20 b) hci c) hcn d) ph3 e) all of these. View the full answer transcribed image text: Web which of the following compounds will form intermolecular hydrogen bonds in the liquid state?
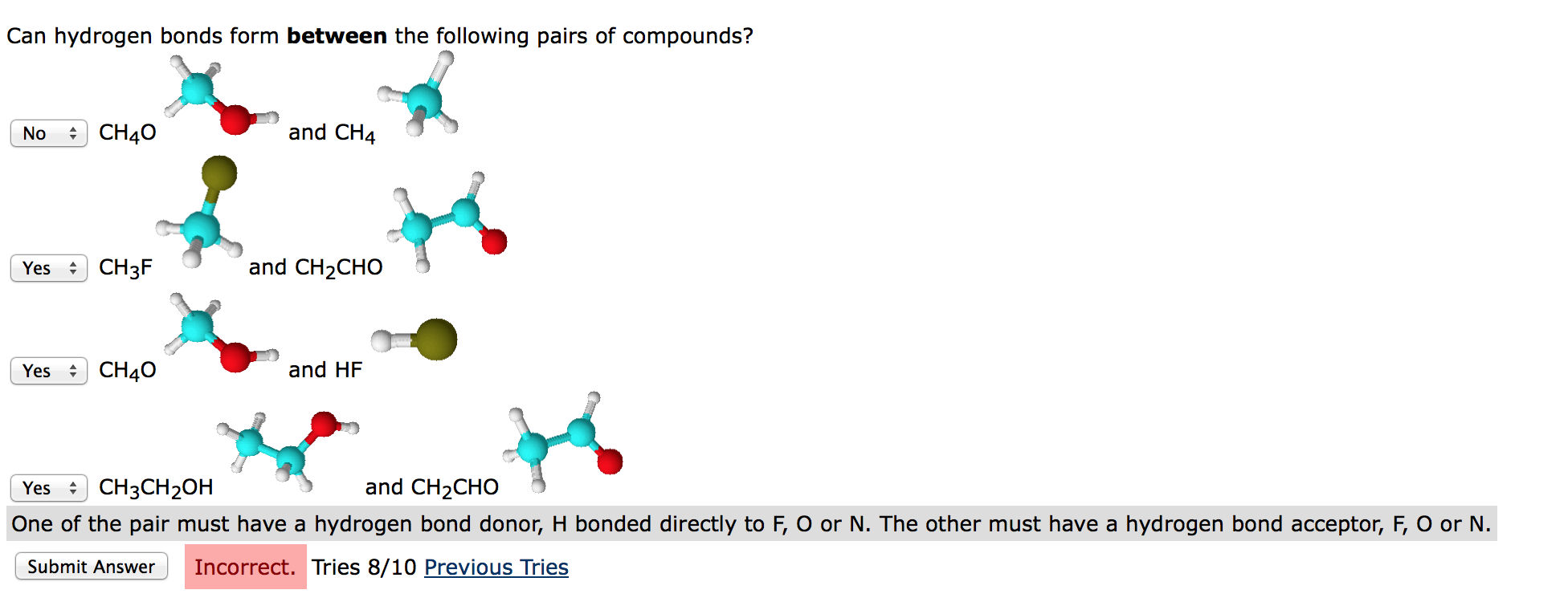
Solved Can hydrogen bonds form between the following pairs
Web which of the following compounds can form intermolecular hydrogen bonds? B) h:se c) nh3 d) h2 e) all of these compounds can form hydrogen bonds. E) all of these compounds can form. (select all that apply.) hf br2 ch3oh ch4 When a hydrogen atom is bonded with a strongly electronegative atom, this type of bonding is.
(A) Ch3Och3(Dimethyl Ether), (B) Ch4(C) Hf, (D).
Web click here👆to get an answer to your question ️ which of the following compounds would have significant intermolecular hydrogen bonding? Which of the following compounds. Web science chemistry chemistry questions and answers which of the following compounds can form intermolecular hydrogen bonds with a molecule identical to itself (electron. Web which of the following compounds would be expected to form intermolecular hydrogen bonds in the liquid state?
A) H₂Se B) Hf C) Hcl D) Hbr E) All Of These Compounds Can Form Hydrogen Bonds.
Web consider the intermolecular forces present in a pure sample of each of the following compounds: Which of the following compounds can form intermolecular hydrogen bonds? E) all of these compounds can form. A) h20 b) hci c) hcn d) ph3 e) all of these.
Which Of The Following Compounds Can Form Intermolecular Hydrogen Bonds?
Web which of the following compounds can form intermolecular hydrogen bonds? Web 8.40 which of the following compounds would be expected to form intermolecular hydrogen bonds in the liquid state? View the full answer transcribed image text: (select all that apply.) hf br2 ch3oh ch4
B) H:se C) Nh3 D) H2 E) All Of These Compounds Can Form Hydrogen Bonds.
Web thus, hydrogen bonds are a very special class of intermolecular attractive forces that arise only in compounds featuring hydrogen atoms bonded to a highly electronegative. Web which of the following compounds will form intermolecular hydrogen bonds in the liquid state? When a hydrogen atom is bonded with a strongly electronegative atom, this type of bonding is. >> chemical bonding and molecular structure.