Which Pair Of Elements Form An Ionic Bond
Which Pair Of Elements Form An Ionic Bond - In general, covalent bonds form. Web ethene contains a carbon/carbon double bond. Web ionic bonds are formed by the combination of positive and negative ions; Web a bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from the other one. Web the pair of elements which on combination are most likely to form an ionic compound is: Potassium (k) and bromine (br) explanation: Ionic bond is formed by complete transfer of electron/s by an atom of electropositive. Web which elements form ionic bonds? A molecule or compound is made when two or more atoms form a chemical bond that links them together. An ionic bond is formed between compounds with a large electronegativity difference between them.
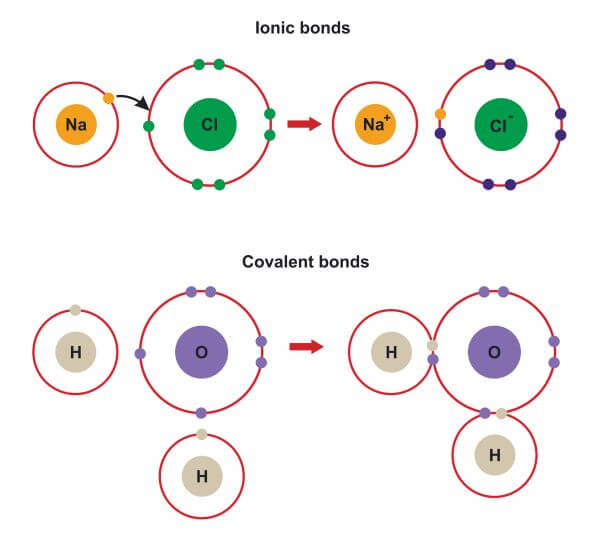
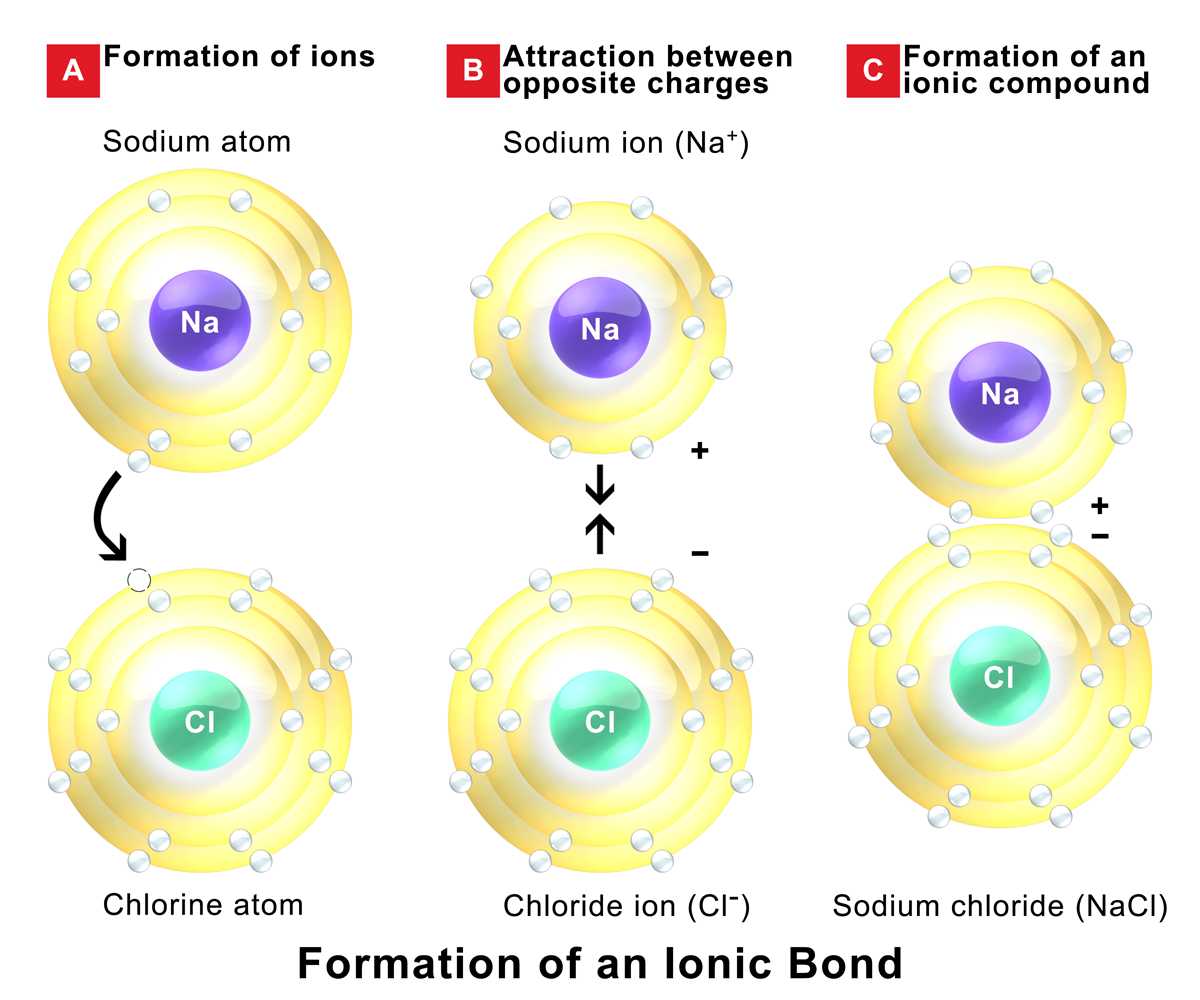
An ionic bond is formed between compounds with a large electronegativity difference between them. Return to bonding menu in modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element). Web 1, which pair of elements is most likely to form an ionic bond. Web which elements form ionic bonds? Potassium (k) and bromine (br) explanation: Web answer 52 people found it helpful podgorica option (2) strontium and chlorine. The combination of these ions form in numerical combinations that generate a neutral (zero charge). Web ionic bonds are formed by the combination of positive and negative ions; Strontium (sr) and chlorine (cl) c. Carbon (c) and oxygen (o) b.
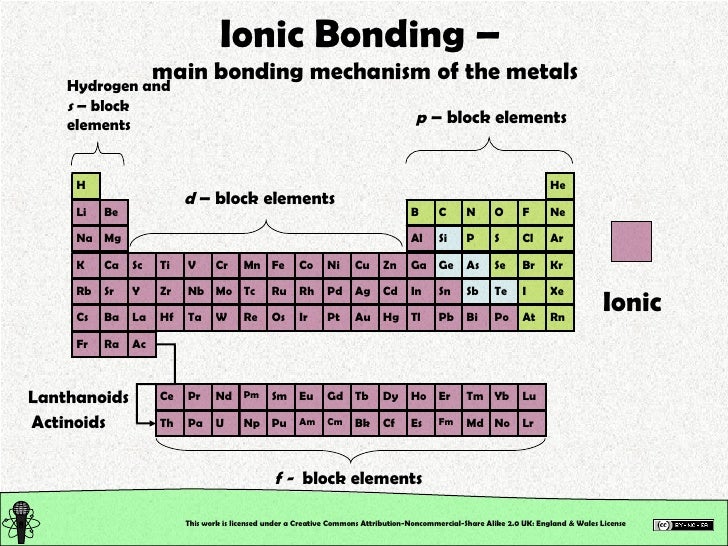
One way to predict the type of bond that forms between two elements is to consider whether each element is a metal or nonmetal. Strontium (sr) and chlorine (cl) c. Both florine and sulfur are non. The combination of these ions form in numerical combinations that generate a neutral (zero charge). Web which pair of elements will form an ionic bond? Web a bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from the other one. Potassium (k) and bromine (br) explanation: In general, covalent bonds form. Web ionic bonding occurs in compounds composed of strongly electropositive elements (metals) and strongly electronegative elements (nonmetals). Sodium is a metal with a low electronegativity it will form an ionic bond with a non metal with a high electronegativity.
Ionic Bond Definition, Types, Properties & Examples
Web answer 52 people found it helpful podgorica option (2) strontium and chlorine. Web a bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from the other one. One way to predict the type of bond that forms between two elements is to consider whether each element.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
The combination of these ions form in numerical combinations that generate a neutral (zero charge). For example, cabr 2 contains a metallic element (calcium, a group 2a metal) and a nonmetallic. Web answer 52 people found it helpful podgorica option (2) strontium and chlorine. Cesium (cs) and germanium (ge) d. One way to predict the type of bond that forms.
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
Both florine and sulfur are non. Return to bonding menu in modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element). Web ethene contains a carbon/carbon double bond. Web which pair of elements would form an ionic bond? Sodium is a metal with a low electronegativity it will form an ionic.
Periodic Table Ions List Periodic Table Timeline
Following this pattern, the triple bond in ethyne molecular formula c 2 h 2, (also known as acetylene, the fuel used in. Web ionic bonds are formed by the combination of positive and negative ions; Return to bonding menu in modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element). Web.
Chemical Structure Chemical Bonding. Ionic, Metallic & Coordinate Bo…
Cesium (cs) and germanium (ge) d. As we have seen, there are. Web the pair of elements which on combination are most likely to form an ionic compound is: Following this pattern, the triple bond in ethyne molecular formula c 2 h 2, (also known as acetylene, the fuel used in. Web comparison of ionic and covalent bonds.
Ionic Bond Examples Biology Dictionary
One way to predict the type of bond that forms between two elements is to consider whether each element is a metal or nonmetal. Web which pair of elements will form an ionic bond? Cesium (cs) and germanium (ge) d. As we have seen, there are. In general, covalent bonds form.
Naming Simple Ionic Compounds Pathways to Chemistry
Return to bonding menu in modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element). One way to predict the type of bond that forms between two elements is to consider whether each element is a metal or nonmetal. Carbon (c) and oxygen (o) b. As we have seen, there are..
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare
Carbon (c) and oxygen (o) b. Web 1, which pair of elements is most likely to form an ionic bond. Web which elements form ionic bonds? Sodium is a metal with a low electronegativity it will form an ionic bond with a non metal with a high electronegativity. Web ethene contains a carbon/carbon double bond.
Ionic Compounds Ionic bonds, Properties, Formation, Examples, Videos
Web which pair of elements will form an ionic bond? In general, covalent bonds form. As we have seen, there are. A molecule or compound is made when two or more atoms form a chemical bond that links them together. Potassium (k) and bromine (br) explanation:
Web Ethene Contains A Carbon/Carbon Double Bond.
Return to bonding menu in modern language, the central idea of an ionic bond is that electrons (one or more, depending on the element). Both florine and sulfur are non. Sodium is a metal with a low electronegativity it will form an ionic bond with a non metal with a high electronegativity. Web ionic bonds are formed by the combination of positive and negative ions;
Web Answer 52 People Found It Helpful Podgorica Option (2) Strontium And Chlorine.
An ionic bond is formed between compounds with a large electronegativity difference between them. Web first, compounds between metal and nonmetal elements are usually ionic. Strontium (sr) and chlorine (cl) c. Web which pair of elements will form an ionic bond?
As We Have Seen, There Are.
For example, cabr 2 contains a metallic element (calcium, a group 2a metal) and a nonmetallic. A molecule or compound is made when two or more atoms form a chemical bond that links them together. Web the pair of elements which on combination are most likely to form an ionic compound is: Web comparison of ionic and covalent bonds.
Web Ionic Bonding Occurs In Compounds Composed Of Strongly Electropositive Elements (Metals) And Strongly Electronegative Elements (Nonmetals).
Web a bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron completely away from the other one. Following this pattern, the triple bond in ethyne molecular formula c 2 h 2, (also known as acetylene, the fuel used in. In general, covalent bonds form. Web which elements form ionic bonds?





.PNG)
/ionic-bond-58fd4ea73df78ca1590682ad.jpg)