Which Pair Of Elements Will Form A Covalent Bond
Which Pair Of Elements Will Form A Covalent Bond - The electrons involved are in the outer shells of the atoms. These electron pairs are known as shared pairs or bonding. The electrons located between the two nuclei are bonding electrons. An atom that shares one or more of its. The classification of covalent bonds is done in three ways, depending on the no. See answer advertisement advertisement brainly user brainly user hydrogen and chlorine. Two electrons or one electron pair constitute a single covalent bond in. The correct option is option c. Web two different atoms can also share electrons and form covalent bonds. Web in order to form a covalent bond, each element has to share one unpaired electron.
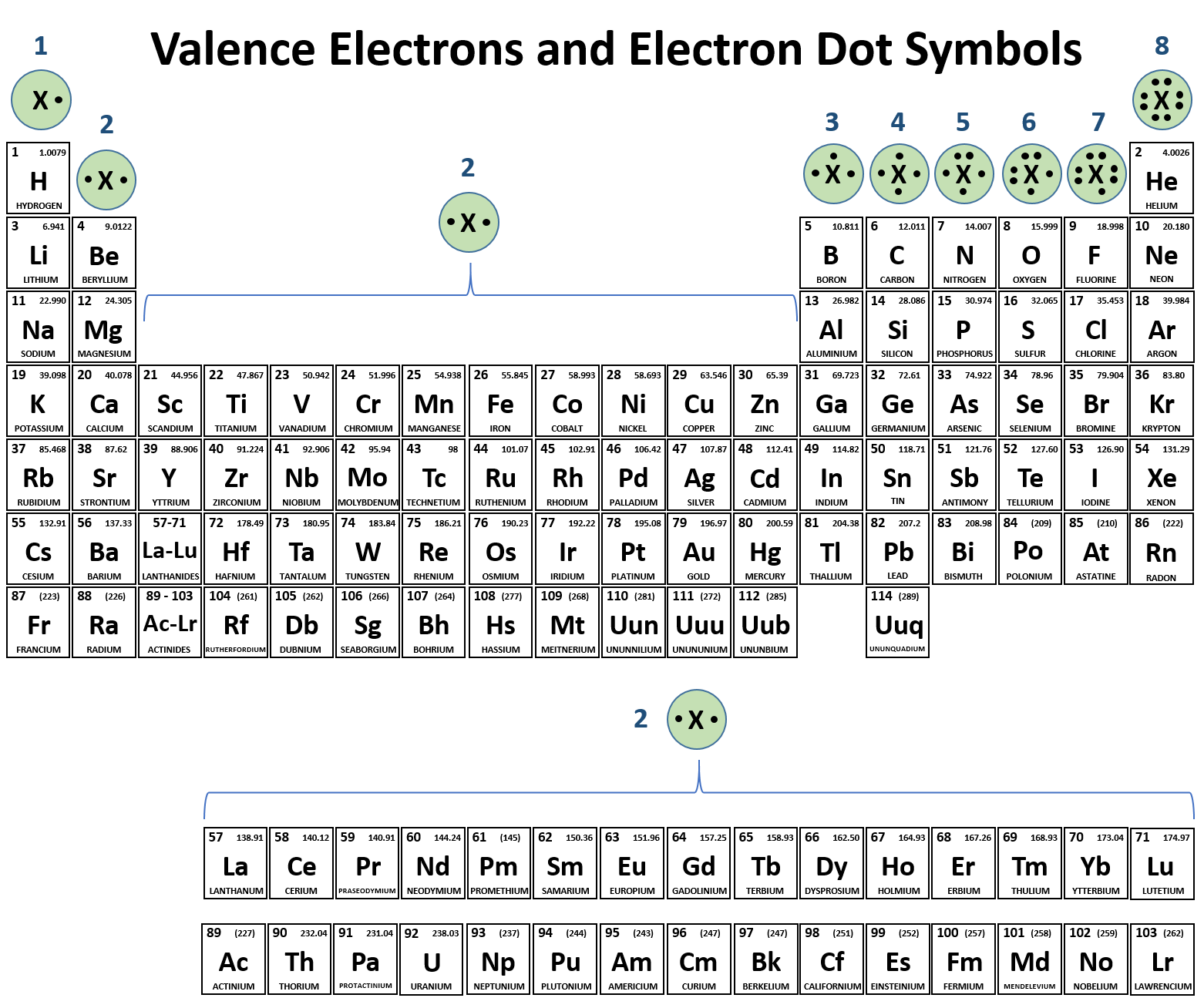
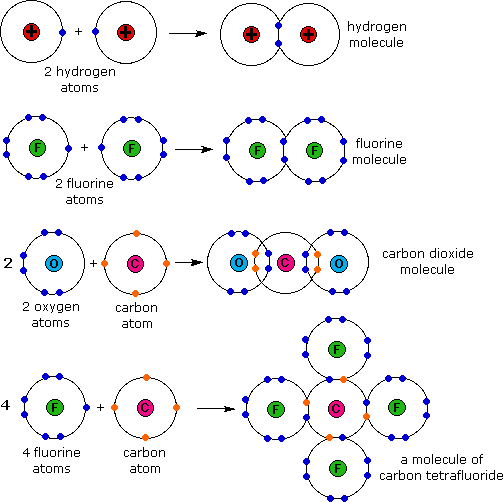
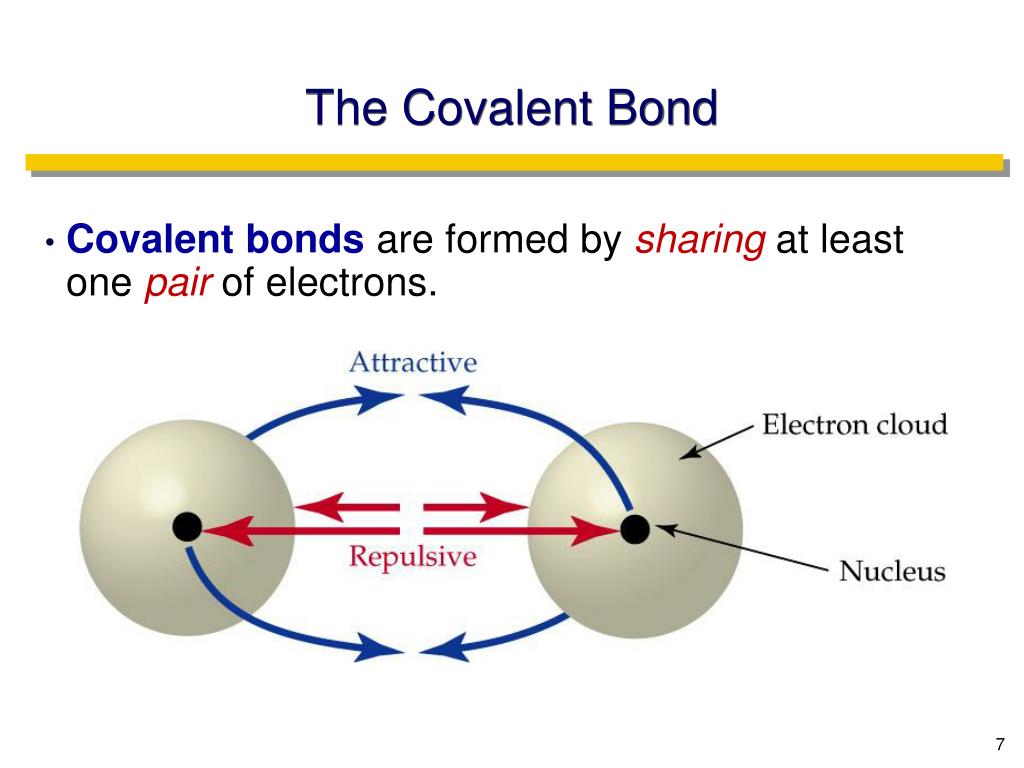
Web a covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. Web a covalent bond is the force of attraction that holds together two atoms that share a pair of valence electrons. Web two different atoms can also share electrons and form covalent bonds. The single electrons match up to make pairs (fig. Web ionic bonds require at least one electron donor and one electron acceptor. Web a covalent bond is formed by the equal sharing of electrons from both participating atoms. Covalent bonds form only between atoms of. Web bonds between two nonmetals are generally covalent; Web covalent bonds are formed only when the electron pair is shared between two atoms. In contrast, atoms with the same electronegativity share electrons in covalent bonds,.
Web covalent bonds are formed only when the electron pair is shared between two atoms. The single electrons match up to make pairs (fig. The correct option is option c. Web two different atoms can also share electrons and form covalent bonds. Web as a general rule, covalent bonds are formed between elements lying toward the right in the periodic table (i.e., the nonmetals). Web bonds between two nonmetals are generally covalent; Web covalent bond is formed by sharing of electron. The oxygen atom forms two. Bonding between a metal and a nonmetal is often ionic. In covalent compounds, atoms form covalent bonds that consist of electron pairs shared between two adjacent atomic nuclei.
Covalent Bonding (Biology) — Definition & Role Expii
Web the sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond are called a bonding pair of electrons. Web bonds between two nonmetals are generally covalent; Web types of covalent bonds. Web in order to form a covalent bond, each element has to share one unpaired electron. The.
Covalent Bond Examples Several Examples of Covalent (molecular) Bonds
An atom that shares one or more of its. Web a covalent bond is formed by the equal sharing of electrons from both participating atoms. The pair of electrons participating in this type of bonding is called a shared pair or. Web a covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Some compounds contain both covalent and ionic bonds. Web a covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. The electrons located between the two nuclei are bonding electrons. Web compounds can be covalent or ionic. Web the sharing of electrons between atoms is called a covalent bond, and the two.
PPT Covalent Bonds PowerPoint Presentation, free download ID6647183
Web a covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. Two electrons or one electron pair constitute a single covalent bond in. Some compounds contain both covalent and ionic bonds. Web the sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in.
IGCSE Chemistry 2017 1.44 Know that a Covalent Bond is Formed Between
Web a covalent bond is formed by the equal sharing of electrons from both participating atoms. Web bonds between two nonmetals are generally covalent; Bonding between a metal and a nonmetal is often ionic. Web a covalent bond is the force of attraction that holds together two atoms that share a pair of valence electrons. Two electrons or one electron.
ASSTUDYPEACH Covalent Bonds Sharing Is Caring!
In contrast, atoms with the same electronegativity share electrons in covalent bonds,. Web covalent bond is formed by sharing of electron. Web compounds can be covalent or ionic. These electron pairs are known as shared pairs or bonding. The single electrons match up to make pairs (fig.
PPT Covalent Bonding PowerPoint Presentation, free download ID4003633
Web in order to form a covalent bond, each element has to share one unpaired electron. An atom that shares one or more of its. The electrons involved are in the outer shells of the atoms. The classification of covalent bonds is done in three ways, depending on the no. In covalent compounds, atoms form covalent bonds that consist of.
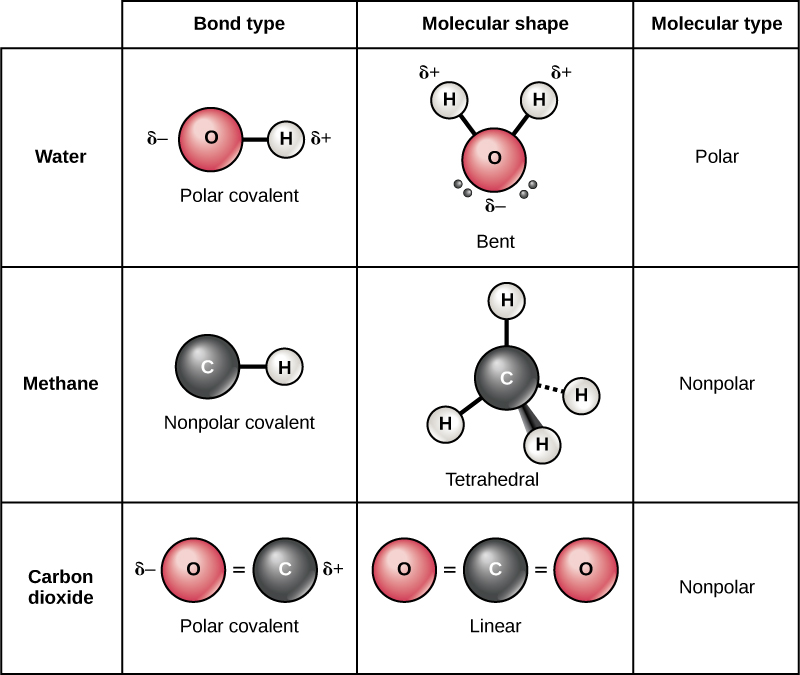
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
Chemical compound is a combination of molecule,. Web covalent bond is formed by sharing of electron. In contrast, atoms with the same electronegativity share electrons in covalent bonds,. The correct option is option c. In covalent compounds, atoms form covalent bonds that consist of electron pairs shared between two adjacent atomic nuclei.
2.2 Chemical Bonds Anatomy & Physiology
The electrons located between the two nuclei are bonding electrons. Web covalent bond is formed by sharing of electron. Web bonds between two nonmetals are generally covalent; Web a covalent bond consists of the simultaneous attraction of two nuclei for one or more pairs of electrons. Web types of covalent bonds.
Atoms, Isotopes, Ions, and Molecules The Building Blocks · Biology
Web ionic bonds require at least one electron donor and one electron acceptor. The electrons located between the two nuclei are bonding electrons. The classification of covalent bonds is done in three ways, depending on the no. Molecules of identical atoms, such as h2 and. The electrons involved are in the outer shells of the atoms.
Web Covalent Bond Is Formed By Sharing Of Electron.
In contrast, atoms with the same electronegativity share electrons in covalent bonds,. For example, water, (\(\ce{h2o}\)), has two covalent bonds between a single oxygen atom. The single electrons match up to make pairs (fig. Covalent bonds form only between atoms of.
Two Electrons Or One Electron Pair Constitute A Single Covalent Bond In.
Web compounds can be covalent or ionic. Web in order to form a covalent bond, each element has to share one unpaired electron. Web bonds between two nonmetals are generally covalent; Web a covalent bond consists of the simultaneous attraction of two nuclei for one or more pairs of electrons.
Chemical Compound Is A Combination Of Molecule,.
Some compounds contain both covalent and ionic bonds. Web which pair of atoms will form a covalent bond? Web covalent bonds are formed only when the electron pair is shared between two atoms. Bonding between a metal and a nonmetal is often ionic.
The Classification Of Covalent Bonds Is Done In Three Ways, Depending On The No.
Web types of covalent bonds. Web a covalent bond is the force of attraction that holds together two atoms that share a pair of valence electrons. The oxygen atom forms two. Some compounds contain both covalent and ionic bonds.