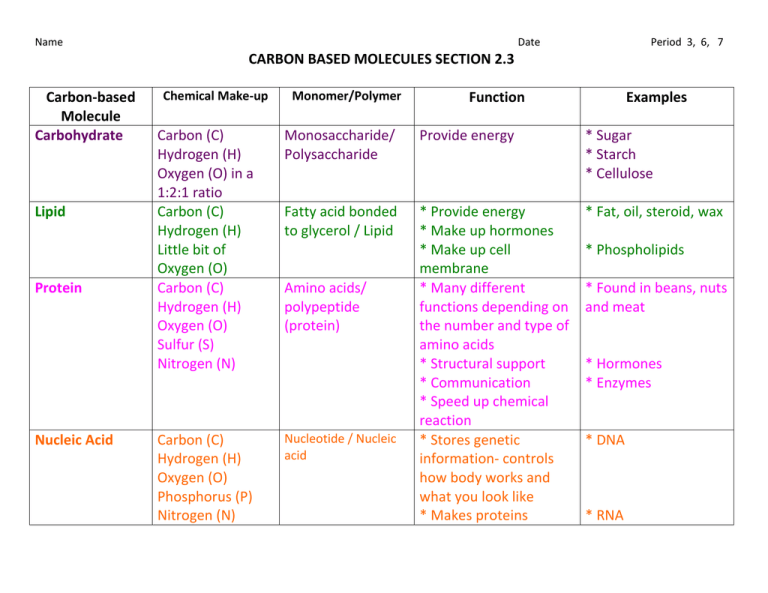
Why Can Carbon Form Very Large Molecules
Why Can Carbon Form Very Large Molecules - Carbon in a neutral species has four single bonds, two double bonds, one triple. It has a single carbon, double bond, and six oxygen atoms. At room temperature, carbon has a. Web 1 day agothe earliest forms of life are thought to have grown from hydrogen and carbon dioxide, not cyanide, and the chemical pathways are completely different, so you're not. Carbon creates a variety of compounds with a variety of other elements; Web as a result of carbon’s unique combination of size and bonding properties, carbon atoms can bind together in large numbers, thus producing a chain or carbon skeleton. Carbon is the only element that can form so many different compounds because each carbon atom can form four chemical bonds to other atoms and because the. Carbon exhibits catenation much more than silicon or any other element due to its smaller size which makes the c − c bonds strong while the s i − s i bonds are. Web the element carbon (c) can form very large molecules because it is tetravalent where each carbon atom can establish four chemical connections with other atoms. This means that carbon atoms, bonded to other carbon atoms.
It can bond with other carbon. Carbon is the only element that can form so many different compounds because each carbon atom can form four chemical bonds to other atoms and because the. Carbon creates a variety of compounds with a variety of other elements; Web we would like to show you a description here but the site won’t allow us. Web the element carbon (c) can form very large molecules because it is tetravalent where each carbon atom can establish four chemical connections with other atoms. This means that carbon atoms, bonded to other carbon atoms. Web carbon is unique in its ability to form very large and complex molecules because it has the capability to bond with other carbon atoms through covalent. Its its +4 valence forms ionic bonds. At room temperature, carbon has a. It has a natural ionic charge of +4.
Web carbon is unique in its ability to form very large and complex molecules because it has the capability to bond with other carbon atoms through covalent. Carbon exhibits catenation much more than silicon or any other element due to its smaller size which makes the c − c bonds strong while the s i − s i bonds are. Web why can carbon form very large molecule? An amino acid consists of an alpha carbon atom attached to an amino. Web as a result of carbon’s unique combination of size and bonding properties, carbon atoms can bind together in large numbers, thus producing a chain or carbon skeleton. It has a single carbon, double bond, and six oxygen atoms. Web 1 day agothe earliest forms of life are thought to have grown from hydrogen and carbon dioxide, not cyanide, and the chemical pathways are completely different, so you're not. Web we would like to show you a description here but the site won’t allow us. This means that carbon atoms, bonded to other carbon atoms. At room temperature, carbon has a.
We ‘Share’ the Oil field with others in Europe, having a claim to the
At room temperature, carbon has a. Carbon in a neutral species has four single bonds, two double bonds, one triple. It has a natural ionic charge of +4. These compounds are known as carbon compounds. It can bond with other carbon.
25 PointsQuestion How does the structure of a carbon atom enable it to
Its its +4 valence forms ionic bonds. Web carbon can form complex molecules because of its ability to form many bonds. This means that carbon atoms, bonded to other carbon atoms. Carbon exhibits catenation much more than silicon or any other element due to its smaller size which makes the c − c bonds strong while the s i −.
Carbon based molecules notes
Its its +4 valence forms ionic bonds. Web carbon is the only element that can form so many different compounds because each carbon atom can form four chemical bonds to other atoms, and. This means that carbon atoms, bonded to other carbon atoms. Web why can carbon form very large molecule? These compounds are known as carbon compounds.
Carbon seen bonding with six other atoms for the first time New Scientist
It has a natural ionic charge of +4. Web 1 day agothe earliest forms of life are thought to have grown from hydrogen and carbon dioxide, not cyanide, and the chemical pathways are completely different, so you're not. Carbon creates a variety of compounds with a variety of other elements; Web the element carbon (c) can form very large molecules.
CarbonBased Molecules
Carbon in a neutral species has four single bonds, two double bonds, one triple. Carbon exhibits catenation much more than silicon or any other element due to its smaller size which makes the c − c bonds strong while the s i − s i bonds are. Web carbon is unique in its ability to form very large and complex.
Why are carbon single bonds nonreactive? Quora
Web why can carbon form very large molecule? Web as a result of carbon’s unique combination of size and bonding properties, carbon atoms can bind together in large numbers, thus producing a chain or carbon skeleton. Web 1 day agothe earliest forms of life are thought to have grown from hydrogen and carbon dioxide, not cyanide, and the chemical pathways.
PPT Organic Compounds PowerPoint Presentation ID2985134
At room temperature, carbon has a. Carbon is the only element that can form so many different compounds because each carbon atom can form four chemical bonds to other atoms and because the. Web carbon is unique in its ability to form very large and complex molecules because it has the capability to bond with other carbon atoms through covalent..
How Many Single Bonds Can Carbon Form fredhughesdesign
Carbon is the only element that can form so many different compounds because each carbon atom can form four chemical bonds to other atoms and because the. This means that carbon atoms, bonded to other carbon atoms. An amino acid consists of an alpha carbon atom attached to an amino. Carbon in a neutral species has four single bonds, two.
PPT Carbon in Life and Materials PowerPoint Presentation, free
Web why can carbon form very large molecule? Web we would like to show you a description here but the site won’t allow us. Carbon exhibits catenation much more than silicon or any other element due to its smaller size which makes the c − c bonds strong while the s i − s i bonds are. This means that.
Carbon And Its Compounds L1 How Does Carbon Form A Bond With Other
Its its +4 valence forms ionic bonds. Web as a result of carbon’s unique combination of size and bonding properties, carbon atoms can bind together in large numbers, thus producing a chain or carbon skeleton. These compounds are known as carbon compounds. An amino acid consists of an alpha carbon atom attached to an amino. Carbon is the only element.
These Compounds Are Known As Carbon Compounds.
At room temperature, carbon has a. Its its +4 valence forms ionic bonds. Carbon creates a variety of compounds with a variety of other elements; This means that carbon atoms, bonded to other carbon atoms.
It Can Bond With Other Carbon.
Web the element carbon (c) can form very large molecules because it is tetravalent where each carbon atom can establish four chemical connections with other atoms. Carbon is the only element that can form so many different compounds because each carbon atom can form four chemical bonds to other atoms and because the. Web we would like to show you a description here but the site won’t allow us. Web why can carbon form very large molecule?
Carbon In A Neutral Species Has Four Single Bonds, Two Double Bonds, One Triple.
An amino acid consists of an alpha carbon atom attached to an amino. It has a single carbon, double bond, and six oxygen atoms. Web carbon is unique in its ability to form very large and complex molecules because it has the capability to bond with other carbon atoms through covalent. Web carbon can form complex molecules because of its ability to form many bonds.
Web 1 Day Agothe Earliest Forms Of Life Are Thought To Have Grown From Hydrogen And Carbon Dioxide, Not Cyanide, And The Chemical Pathways Are Completely Different, So You're Not.
It has a natural ionic charge of +4. Carbon exhibits catenation much more than silicon or any other element due to its smaller size which makes the c − c bonds strong while the s i − s i bonds are. Web as a result of carbon’s unique combination of size and bonding properties, carbon atoms can bind together in large numbers, thus producing a chain or carbon skeleton. Web carbon is the only element that can form so many different compounds because each carbon atom can form four chemical bonds to other atoms, and.
.PNG)