Why Do The Noble Gases Not Form Compounds Readily
Why Do The Noble Gases Not Form Compounds Readily - They do not gain or loose electrons to form ions what is the charge on an atom? Web the compound in atlanta. Noble gases are more _____ than other elements because they. Good conductors of heat d. Web although noble gases do not normally react with other elements to form compounds, there are some exceptions. Web all noble gases have full s and p outer electron shells (except helium, which has no p sublevel), and so do not form chemical compounds easily. A.they have empty outer energy levels b.they have no electrons c.they have seen electron in the outer energy level. Mixtures d in general, nonmetals are __. Web they traditionally have been labeled group 0 in the periodic table because for decades after their discovery it was believed that they could not bond to other atoms;. To become more chemically stable, an atom that has two electrons in.
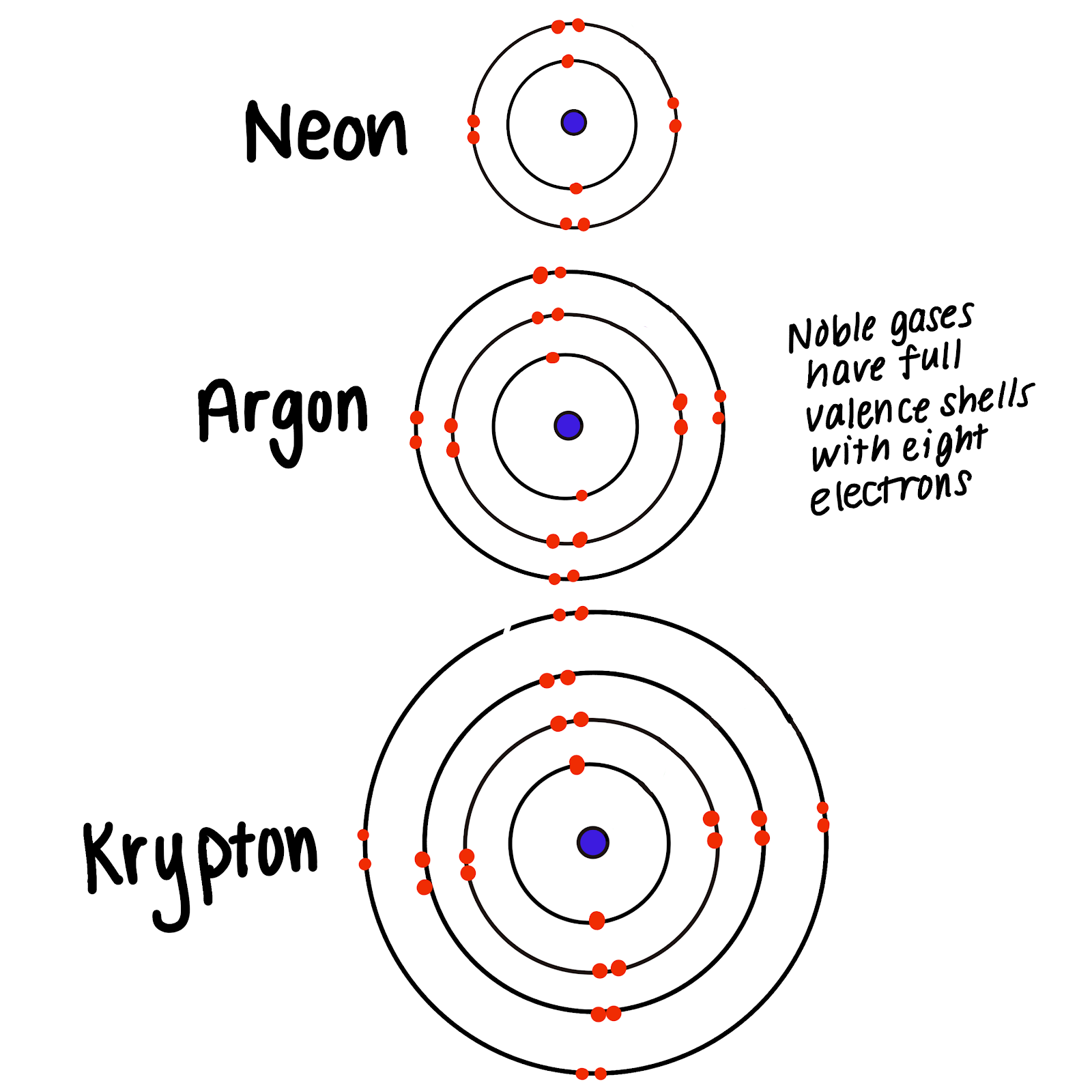
Web why noble gases do not readily form compounds? Xe may form compounds with fluoride and. Their outer energy levels have 8 valence electrons. Web the compound in atlanta. A.they have empty outer energy levels b.they have no electrons c.they have seen electron in the outer energy level. Web why don't the noble gases form compounds readily? Web they traditionally have been labeled group 0 in the periodic table because for decades after their discovery it was believed that they could not bond to other atoms;. Lining up plans in atlanta? Web all noble gases have full s and p outer electron shells (except helium, which has no p sublevel), and so do not form chemical compounds easily. Web although noble gases do not normally react with other elements to form compounds, there are some exceptions.
Xe may form compounds with fluoride and. Their outer energy levels have 8 valence electrons. Web why are the noble gases inert? Web why don't the noble gases form compounds readily? Web all noble gases have full s and p outer electron shells (except helium, which has no p sublevel), and so do not form chemical compounds easily. Web they traditionally have been labeled group 0 in the periodic table because for decades after their discovery it was believed that they could not bond to other atoms;. Web why do the noble gases not form compounds readily? Mixtures d in general, nonmetals are __. Web why do the noble gases not form compounds readily? Copy and paste are not allow.
Why Do Noble Gases Not React WHYPLJ
Noble gases are more _____ than other elements because they. Web why do the noble gases not form compounds readily? Atoms form compounds when the compound is more _____ than the separate atoms. Web why noble gases do not readily form compounds? Copy and paste are not allow.
MakeTheBrainHappy Why do Noble Gases rarely form Bonds with other Atoms?
Xe may form compounds with fluoride and. Web why do the noble gases not form compounds readily? A.they have empty outer energy levels b.they have no electrons c.they have seen electron in the outer energy level. Explain why the noble gases do not form. Atoms form compounds when the compound is more _____ than the separate atoms.
Reactions Impossible How Chemists Strived to Make Noble Gas Compounds
Their outer energy levels have 8 valence electrons. Xe may form compounds with fluoride and. Web why do the noble gases not form compounds readily? They do not gain or loose electrons to form ions what is the charge on an atom? Good conductors of heat d.
Why do noble gases have large atomic radii Part 47P blockUnit 7I
A.they have empty outer energy levels b.they have no electrons c.they have seen electron in the outer energy level. Their outer energy levels are completely filled with electrons what is the number of pottasium atoms compared to. The clathrates , first described in. To become more chemically stable, an atom that has two electrons in. Xe may form compounds with.
Noble Gas Chemical Compounds
Their outer energy levels are completely filled with electrons what is the number of pottasium atoms compared to. Copy and paste are not allow. 0 ionic bonds form nonmetals and metals in an ionic bond the. Web all noble gases have full s and p outer electron shells (except helium, which has no p sublevel), and so do not form.
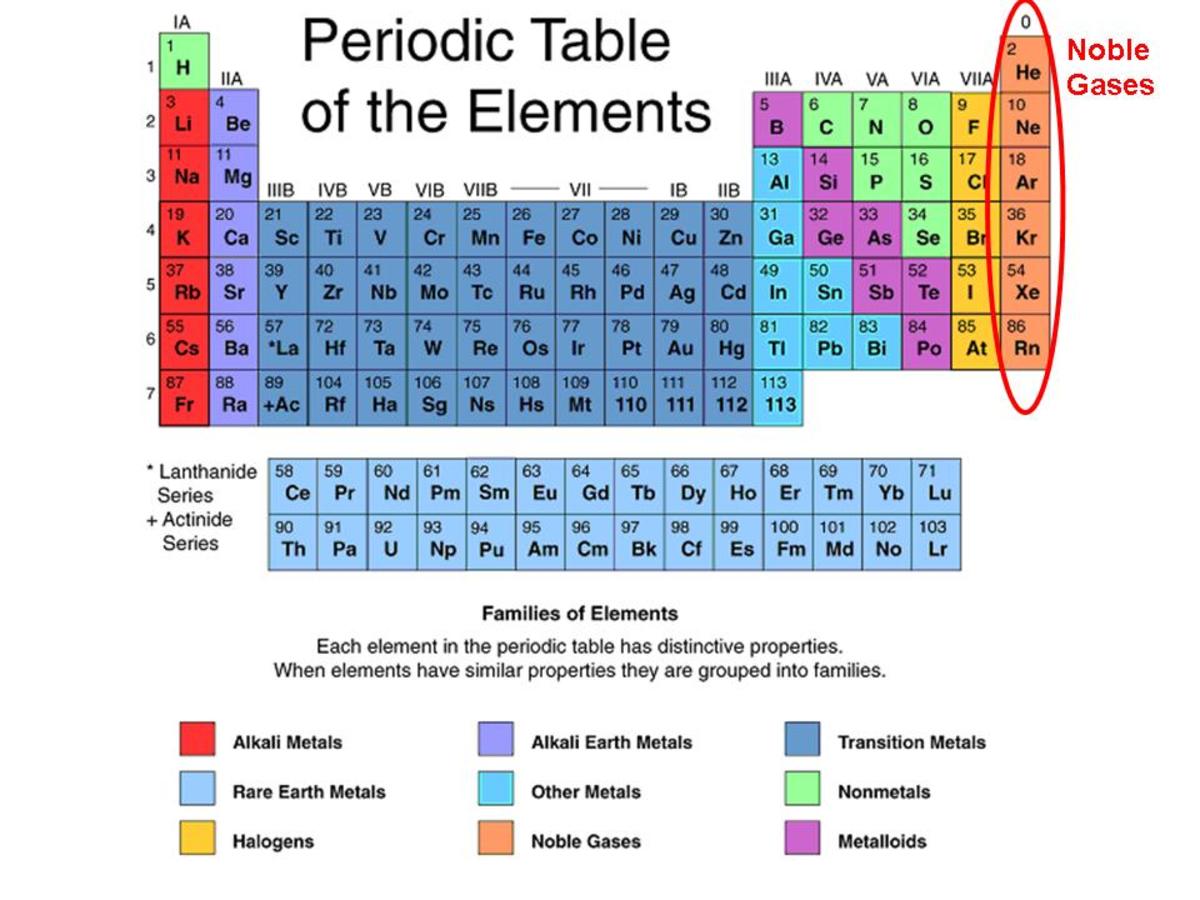
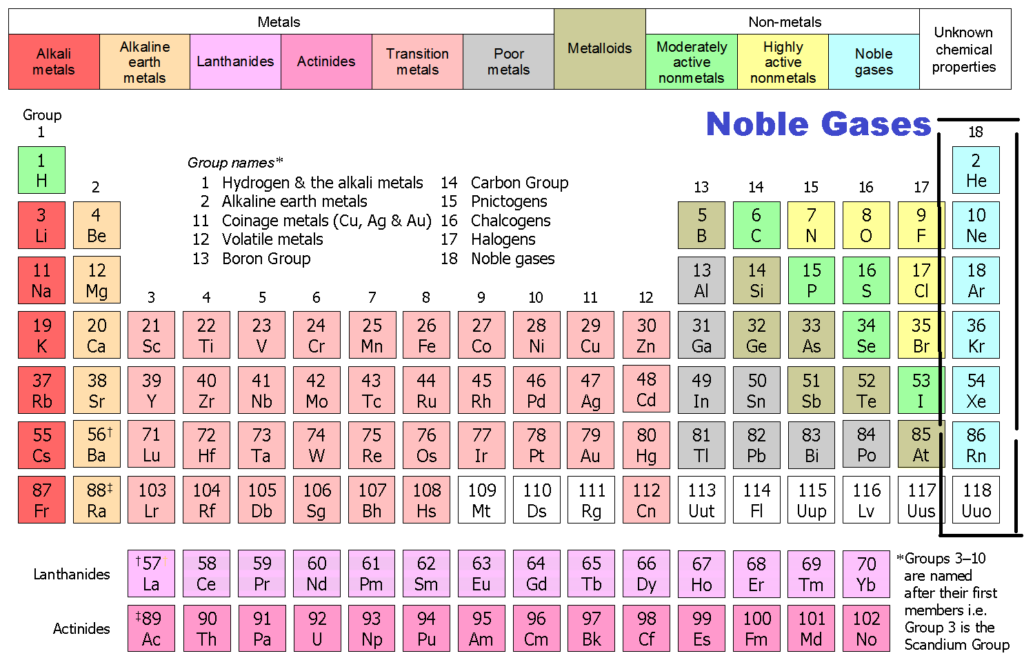
Group 18 The Noble Gases
Web although noble gases do not normally react with other elements to form compounds, there are some exceptions. Good conductors of heat d. Lining up plans in atlanta? A.they have empty outer energy levels b.they have no electrons c.they have seen electron in the outer energy level. Copy and paste are not allow.
What's So Noble About Noble Gases? Owlcation
Loved by our community 106 people found it helpful raminder1 because they are already in stable state. Web the compound in atlanta. Copy and paste are not allow. Lining up plans in atlanta? Web why noble gases do not readily form compounds?
What Is The Reactivity Of Noble Gases howtogetalaid
0 ionic bonds form nonmetals and metals in an ionic bond the. Web all noble gases have full s and p outer electron shells (except helium, which has no p sublevel), and so do not form chemical compounds easily. Mixtures d in general, nonmetals are __. Explain why the noble gases do not form. Atoms form compounds when the compound.
What Are Noble Gases? Definition and Properties
Web all noble gases have full s and p outer electron shells (except helium, which has no p sublevel), and so do not form chemical compounds easily. Web all noble gases have full s and p outer electron shells (except helium, which has no p sublevel), and so do not form chemical compounds easily. Xe may form compounds with fluoride.
What Is The Reactivity Of Noble Gases howtogetalaid
Atoms form compounds when the compound is more _____ than the separate atoms. Web why do the noble gases not form compounds readily? Copy and paste are not allow. Web why noble gases do not readily form compounds? 0 ionic bonds form nonmetals and metals in an ionic bond the.
Web Why Are The Noble Gases Inert?
Gases at room temperature d in general,. Good conductors of electricity b. Explain why the noble gases do not form. Web all noble gases have full s and p outer electron shells (except helium, which has no p sublevel), and so do not form chemical compounds easily.
Web All Noble Gases Have Full S And P Outer Electron Shells (Except Helium, Which Has No P Sublevel), And So Do Not Form Chemical Compounds Easily.
Noble gases are more _____ than other elements because they. Good conductors of heat d. Xe may form compounds with fluoride and. Web although noble gases do not normally react with other elements to form compounds, there are some exceptions.
Mixtures D In General, Nonmetals Are __.
Web why do the noble gases not form compounds readily? A.they have empty outer energy levels b.they have no electrons c.they have seen electron in the outer energy level. Noble gases do not form compounds readily as noble gases have their outermost orbit completely filled and have a stable configuration. Loved by our community 106 people found it helpful raminder1 because they are already in stable state.
The Clathrates , First Described In.
Web the compound in atlanta. Copy and paste are not allow. They do not gain or loose electrons to form ions what is the charge on an atom? Web they traditionally have been labeled group 0 in the periodic table because for decades after their discovery it was believed that they could not bond to other atoms;.
.PNG)



:max_bytes(150000):strip_icc()/Xenonhexafluoride-56a12d265f9b58b7d0bccc78.png)

.PNG)