In What Form Can An Ionic Compound Conduct Electricity
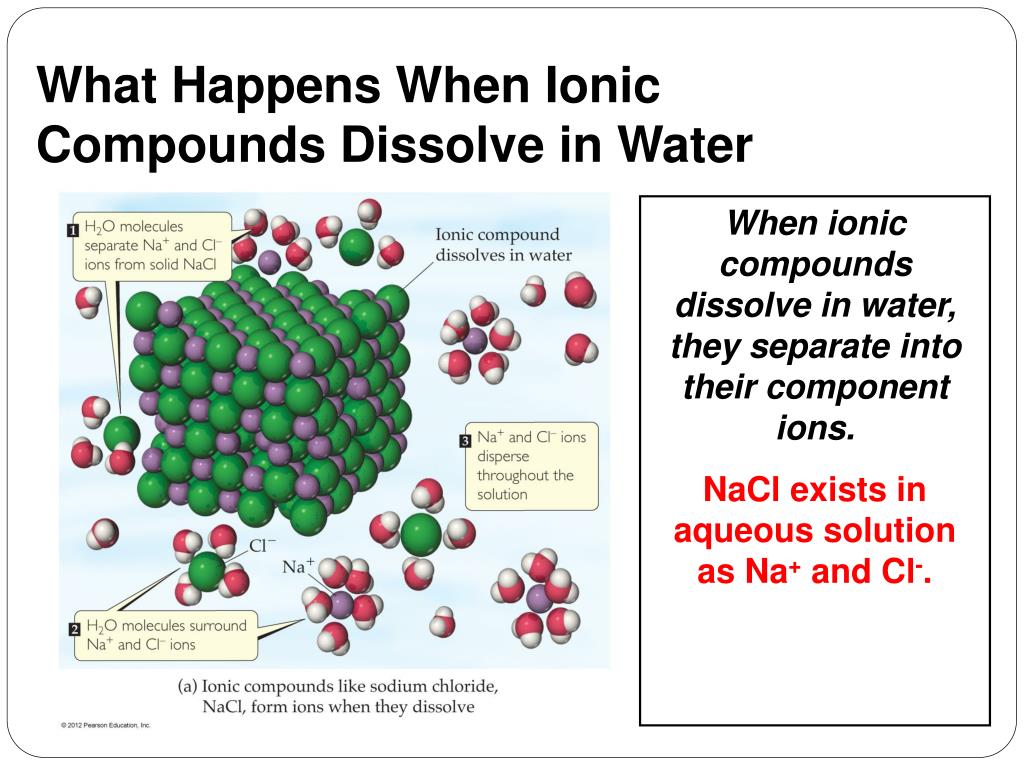
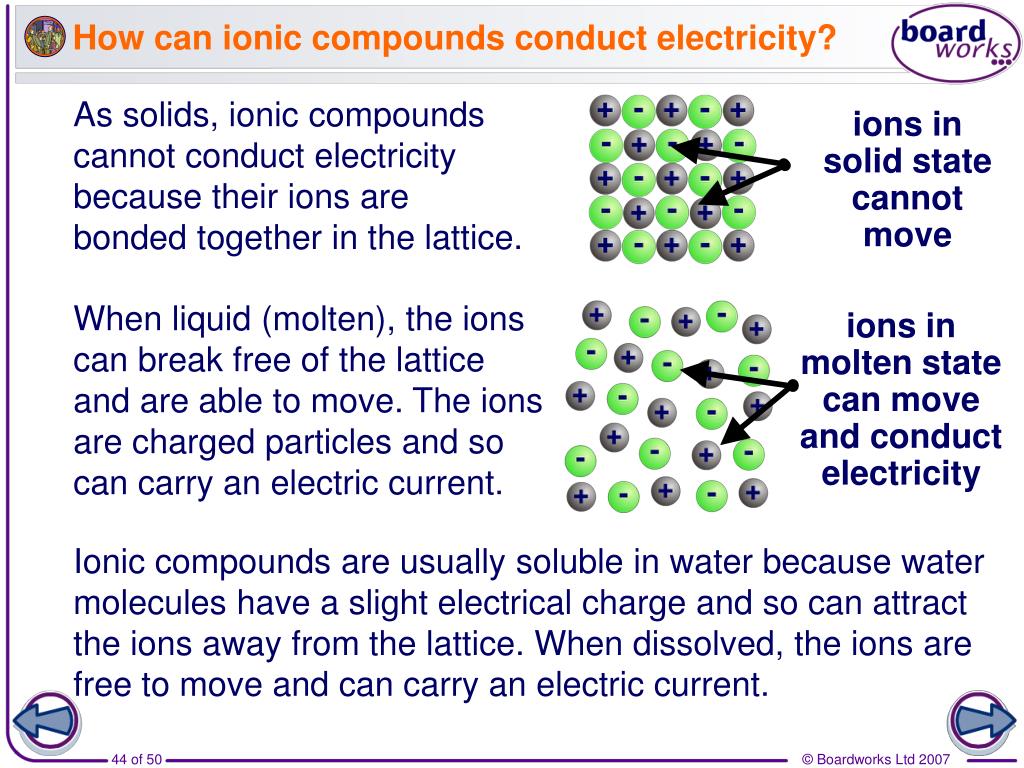
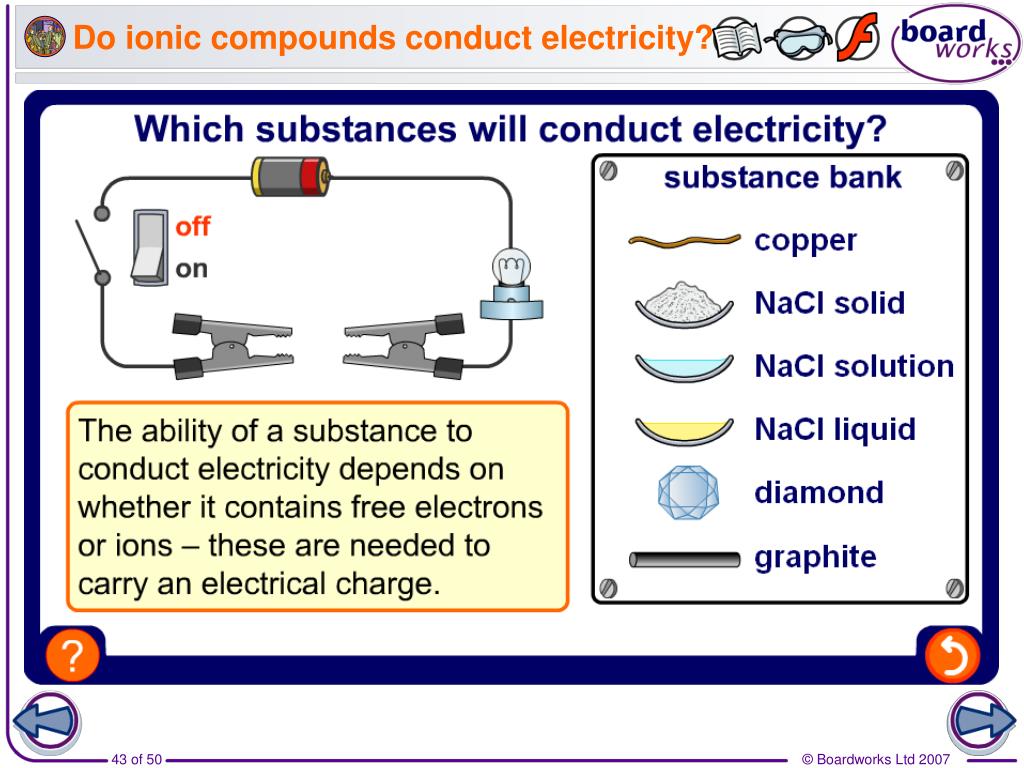
In What Form Can An Ionic Compound Conduct Electricity - Web in molten form, ionic compounds conduct an electric current as ions are available for conduction of electricity. Web ionic compounds conduct electricity in molten or aqueous form; Melting an ionic compound also frees the ions to conduct a. Web cations move to one electrode, while anions move to the other, allowing electricity to flow (see figure below ). Web are there ionic solids that conduct electricity? Web in solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles). Ionic compounds conduct electricity when molten or in solution. Web in general, covalent network substances do not conduct electricity. Ask question asked 3 years, 4 months ago modified 3 years, 3 months ago viewed 621 times 4 we are taught in. Ionic compounds also conduct electricity in aqueous solution.
This is because both processes make their ions free to move. Covalent compounds do not conduct electricity in any form; Off vibrating atoms in the crystal lattice. Luckyllaher1265 luckyllaher1265 05.12.2022 chemistry secondary. Ionic compounds can conduct electricity when dissolved in water, since the ions dissociate, current can travel through the solution. Web in solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles). This is because they do not have charged particles which are free to move. Ask question asked 3 years, 4 months ago modified 3 years, 3 months ago viewed 621 times 4 we are taught in. Web ionic compounds conduct electricity in molten or aqueous form; In a giant ionic lattice, there are strong electrostatic forces of attraction.
Web find an answer to your question in what form can an ionic compound conduct electricity. Melting an ionic compound also frees the ions to conduct a. Ionic compounds can conduct electricity when they are dissolved in. Ionic solids (such as hcl and nacl) dissolved in water conduct electricty due to the dissociation of the ionic components. Ionic solids do not conduct because the atoms are typically bound too tightly to a crystal lattice for anything to function as mobile charge carriers. Web reactive metals are extracted from their ores using electrolysis. Luckyllaher1265 luckyllaher1265 05.12.2022 chemistry secondary. Web are there ionic solids that conduct electricity? Web in solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles). Off vibrating atoms in the crystal lattice.
IGCSE Chemistry 2017 1.56C Understand Why Ionic Compounds Conduct
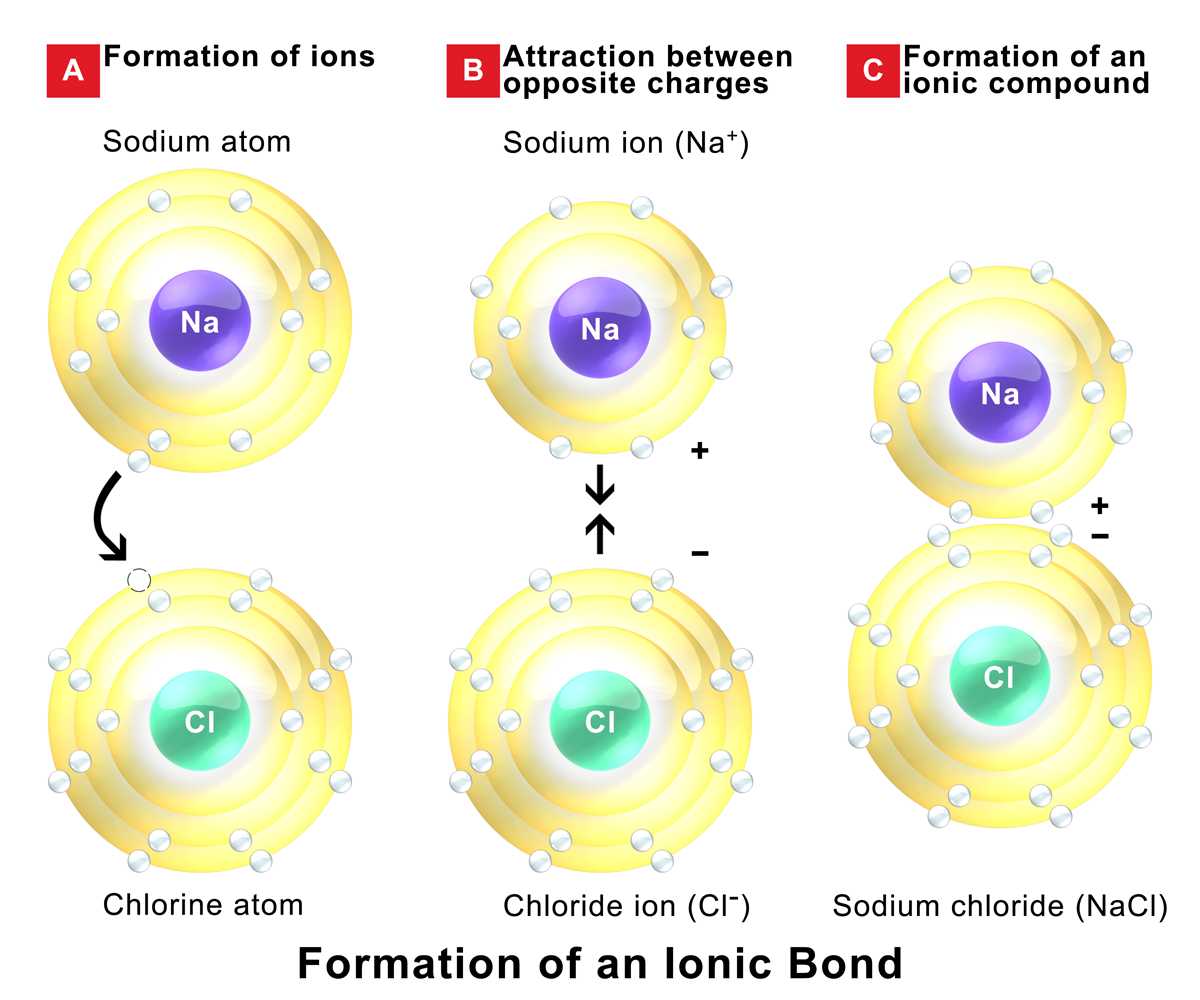
Metals give up electrons and therefore become positive charged. Ionic compounds also conduct electricity in aqueous solution. Ionic compounds are made from metallic elements bonded to nonmetallic elements. Web ionic compounds conduct electricity in molten or aqueous form; Web ionic compounds are conductors of electricity when they are in a molten state or aqueous state.
PPT UNIT 5 PowerPoint Presentation, free download ID6635190
Metals give up electrons and therefore become positive charged. Web cations move to one electrode, while anions move to the other, allowing electricity to flow (see figure below ). Ionic compounds conduct electricity when molten or in solution. Web ionic compounds are conductors of electricity when they are in a molten state or aqueous state. Luckyllaher1265 luckyllaher1265 05.12.2022 chemistry secondary.
Chemical Bonding (Electrical Conductivity of Ionic Compound) Concept
Ask question asked 3 years, 4 months ago modified 3 years, 3 months ago viewed 621 times 4 we are taught in. Web tap water, due to impurities, does. Ionic solids do not conduct because the atoms are typically bound too tightly to a crystal lattice for anything to function as mobile charge carriers. Ionic compounds can conduct electricity when.
PPT What are bonds? PowerPoint Presentation, free download ID5980343
Ionic solids (such as hcl and nacl) dissolved in water conduct electricty due to the dissociation of the ionic components. Web cations move to one electrode, while anions move to the other, allowing electricity to flow (see figure below ). Off vibrating atoms in the crystal lattice. Ionic compounds also conduct electricity in aqueous solution. Melting an ionic compound also.
Lecture 7.2 Ionic Compounds
Web find an answer to your question in what form can an ionic compound conduct electricity. Web tap water, due to impurities, does. Web ionic compounds are conductors of electricity when they are in a molten state or aqueous state. Metals give up electrons and therefore become positive charged. Ionic compounds conduct electricity when molten or in solution.
ASSTUDYPEACH The Name’s Bond Ionic Bond.
Ionic compounds can conduct electricity when they are dissolved in. Ionic compounds are made from metallic elements bonded to nonmetallic elements. Ask question asked 3 years, 4 months ago modified 3 years, 3 months ago viewed 621 times 4 we are taught in. Web in molten form, ionic compounds conduct an electric current as ions are available for conduction of.
PPT What are bonds? PowerPoint Presentation, free download ID5980343
Web in solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles). Covalent compounds do not conduct electricity in any form; Ask question asked 3 years, 4 months ago modified 3 years, 3 months ago viewed 621 times 4 we are taught in. Ionic compounds are made.
PPT IV. Chemical Bonding PowerPoint Presentation, free download ID
This is because both processes make their ions free to move. Web in general, covalent network substances do not conduct electricity. Off vibrating atoms in the crystal lattice. Web ionic compounds are conductors of electricity when they are in a molten state or aqueous state. Web ionic compounds conduct electricity when molten to form a liquid or dissolved in water.
Ionic Compounds Ionic bonds, Properties, Formation, Examples, Videos
Metals give up electrons and therefore become positive charged. Web tap water, due to impurities, does. Web 1 2 3 4 properties of ionic compounds ionic compounds have regular structures, called giant ionic lattices. Web are there ionic solids that conduct electricity? Ionic solids (such as hcl and nacl) dissolved in water conduct electricty due to the dissociation of the.
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare
Web tap water, due to impurities, does. Web ionic compounds conduct electricity in molten or aqueous form; Web 1 2 3 4 properties of ionic compounds ionic compounds have regular structures, called giant ionic lattices. Metals give up electrons and therefore become positive charged. Ionic compounds conduct electricity when molten or in solution.
Web In Molten Form, Ionic Compounds Conduct An Electric Current As Ions Are Available For Conduction Of Electricity.
Web ionic compounds are conductors of electricity when they are in a molten state or aqueous state. Web ionic compounds conduct electricity in molten or aqueous form; Luckyllaher1265 luckyllaher1265 05.12.2022 chemistry secondary. Web ionic compounds conduct electricity when molten to form a liquid or dissolved in water to form an aqueous solution.
Covalent Compounds Do Not Conduct Electricity In Any Form;
Ask question asked 3 years, 4 months ago modified 3 years, 3 months ago viewed 621 times 4 we are taught in. Web in solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles). Off vibrating atoms in the crystal lattice. Web tap water, due to impurities, does.
In A Giant Ionic Lattice, There Are Strong Electrostatic Forces Of Attraction.
Web find an answer to your question in what form can an ionic compound conduct electricity. Ionic compounds can conduct electricity when dissolved in water, since the ions dissociate, current can travel through the solution. Web are there ionic solids that conduct electricity? Web reactive metals are extracted from their ores using electrolysis.
(2023) A Team Of Researchers Claims To Have Created The First Materials That Conduct Electricity Perfectly At Room.
Ionic compounds conduct electricity when molten or in solution. Ionic solids do not conduct because the atoms are typically bound too tightly to a crystal lattice for anything to function as mobile charge carriers. This is because they do not have charged particles which are free to move. Web in solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles).




.PNG)